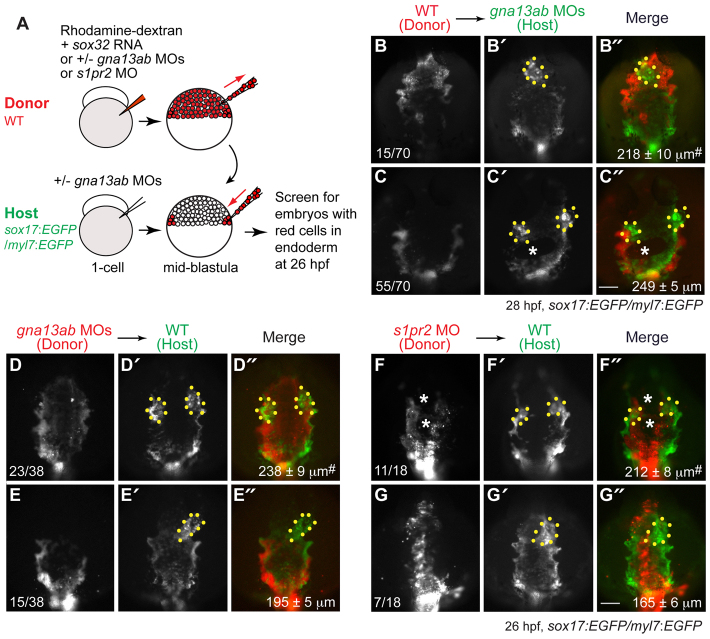
Fig. 8.
S1pr2/Gα13 signaling functions autonomously within the endoderm to regulate myocardial migration. (A) The cell transplantation procedures. Sox32-overexpressing and rhodamine-dextran labeled wild-type or gna13ab or s1pr2 morphant donor cells were transplanted into Tg(sox17:EGFP)/(myl7:EGFP) host embryos. In the case of hosts to be injected with wild-type donor cells, the embryos were injected with gna13ab MOs. (B-G″) Epifluorescence images of the anterior region of Tg(sox17:EGFP)/(myl7:EGFP) hosts at 26-28 hpf, showing the morphology of the anterior endoderm and cardiomyocytes. (B-C″) Wild-type donor cells were transplanted into gna13ab morphant hosts. (D-E″) gna13ab morphant donor cells were transplanted into wild-type hosts. (F-G″) s1pr2 morphant donor cells were transplanted into wild-type hosts. (B-G) Rhodamine-dextran labeling donor cells. (B′-G′) GFP-expressing anterior endoderm and cardiomyocytes. (B″-G″) Merged images of B′-G′ and B-G. Dorsoanterior view with anterior upwards; yellow dots indicate cardiomyocytes; white asterisks indicate endodermal holes; the number of embryos is indicated at the bottom left of the ‘donor’ panel; average endodermal width is shown at the bottom right of the ‘merge’ panel; #P<0.001 versus the corresponding control groups. Scale bars: 100 μm.