Table 2.
| Entry | Diyne | Nitrile | Product | Yieldc |
|---|---|---|---|---|
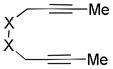 X = C(CO2Et)2 |
|
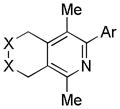
|
||
| 1 | 1 | a (Ar = Ph) | 1a | 98% |
| 2 | 1 | b (Ar = 2-Me-Ph) | 1b | 87% |
| 3 | 1 | c (Ar = 4-OMe-Ph) | 1c | 90% |
| 4 | 1 | d (Ar = 4-CF3-Ph) | 1d | 99% |
| 5 | 1 | e (Ar = 3,5-F2-Ph) | 1e | >99% |
| 6 | 1 | f (Ar = 2-Naphthyl) | 1f | 96% |
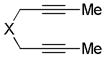 X = C(CO2Me)2 |
|
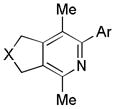
|
||
| 7 | 2 | a (Ar = Ph) | 2a | 92% (86%)d |
| 8 | 2 | b (Ar = 2-Me-Ph) | 2b | 85% (81%)d |
| 9 | 2 | c (Ar = 4-OMe-Ph) | 2c | 89% (64%)d |
| 10 | 2 | d (Ar = 4-CF3-Ph) | 2d | 98% (94%)d |
| 11 | 2 | e (Ar = 3,5-F2-Ph) | 2e | >99% |
|
|

|
|||
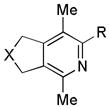
| 12 | 2 | g | 2g | >99%e |
|
|
|
|||
| 13 | 2 | h (R = Me) | 2h | 94% (69%)d |
| 14 | 2 | i (R = i-Pr) | 2i | 90% (72%)d |
|
||||
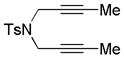
| 15 | 3 | a | 3a | 73% (78%)d |
|
||||
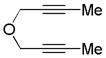
| 16 | 4 | a | 4a | 80% (93%)d |
|
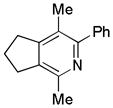
|
|||
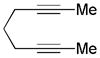
| 17 | 5 | a | 5a | 93% |
Reaction Conditions: 3 mol % Ni(COD)2, 3 mol % Xantphos, diyne (1 equiv., 0.1 M), nitrile (1.5 equiv.), toluene, RT, 3 h.
Nitriles were neither distilled nor degassed. Nitriles were used as received.
Isolated yields.
Yields from reference 6a.
The reaction was run at 100 °C.
