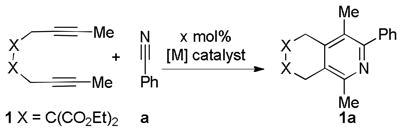
Table 3.
Comparison of Metal-Catalysts for Cycloaddition of Diynes and Nitrile.
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | [M] Catalyst x mol% | equiv. of Nitrile a | Temp | Time | Conva 1 | Yielda,b 1a | ||
| 1 | 5 mol% [Co]c | 20 | RT | 24h | 86% | 70% | ||
| 2 | 3 mol% [Rh]d | 1.5 | 60 °C | 3h | >99% | >99% | ||
| 3 | 3 mol% [Rh]e | 1.5 | RT | 3h | 32% | 15% | ||
| 4 | 10 mol% [Ru]f | 1.5 | 60 °C | 24h | 6%g | - | ||
| 5 | 3 mol% [Ni/SIPr]h | 1.5 | RT | 3h | >99% | 80% | ||
| 6 | 3 mol% [Ni/Xant]i | 1.5 | RT | 3h | >99% | >99% (98%) | ||
Analysed by GC using naphthalene as an internal standard.
Isolated yield in parenthesis.
5 mol% CoCl2, 6 mol% DPPE, xs Zn, diyne (1 equiv., 1 M), nitrile (20 equiv), NMP, RT.
3 mol% [Rh(COD)]BF4, 3 mol% Segphos, diyne (1equiv., 0.1 M), nitrile (1.5 equiv.), DCE, 60 °C.
Same conditions as in [d], but the reaction was run @ RT.
10 mol% Cp*Ru(COD)Cl, diyne (1 equiv., 0.1 M), nitrile (1.5 equiv.), DCE, 60 °C, 24 h.
Analysed by NMR.
3 mol% Ni(COD)2, 3 mol% SIPr, diyne (1 equiv., 0.1 M), nitrile (1.5 equiv.), THF, RT.
3 mol% Ni(COD)2, 3 mol% Xantphos, diyne (1 equiv., 0.1 M), nitrile (1.5 equiv.), toluene, RT.