Table 11. Single-Photon and Two-Photon Uncaging Quantum Efficiencies of Photolabile Protecting Groups.
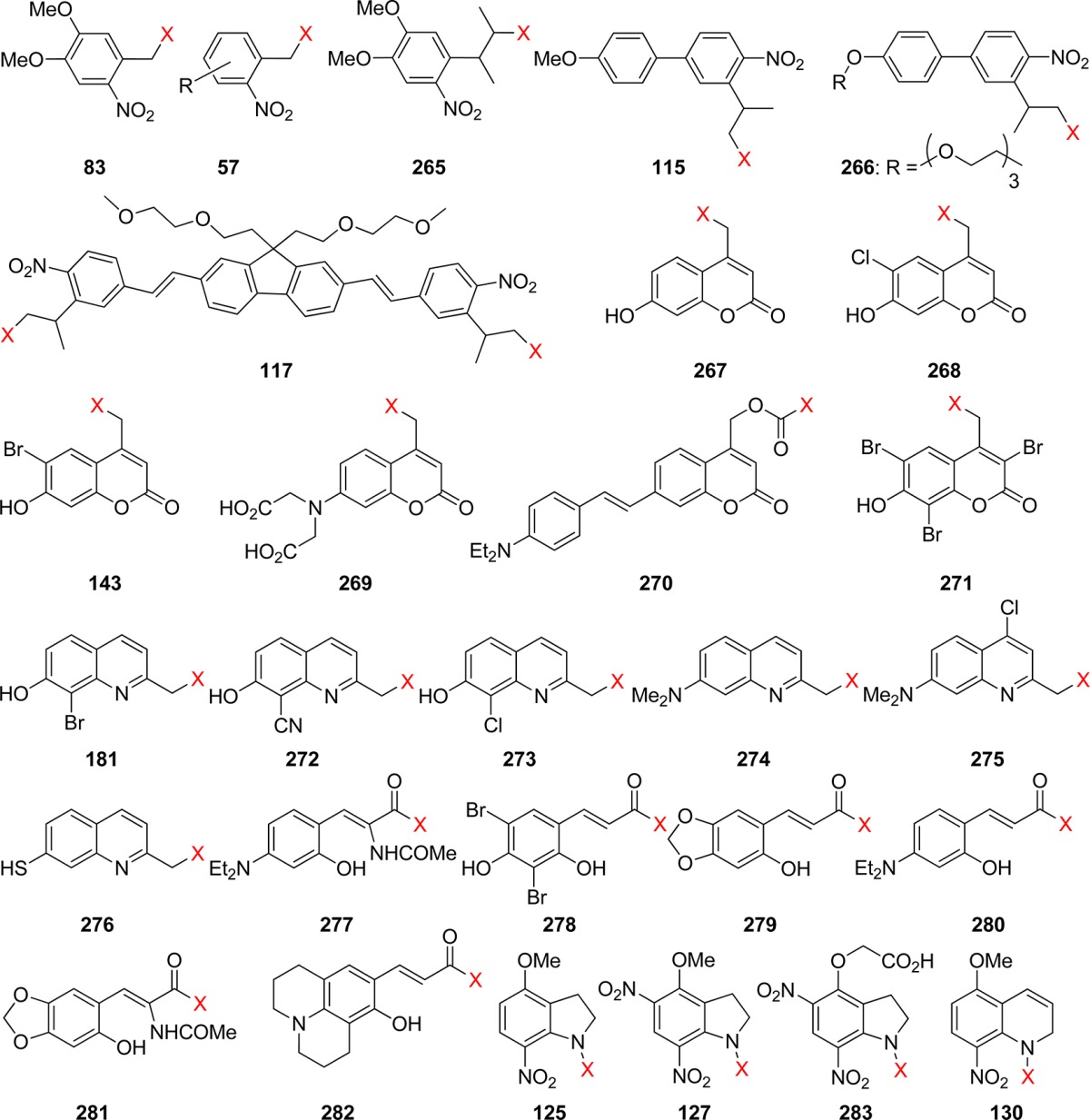
| PPG | Φa | λ/nm | δunc/GMb | λ/nm | ref |
|---|---|---|---|---|---|
| 83 (4,5-dimethoxy-2-nitrobenzyl, NV) | 0.16 | 305 | 0.035c | 730 | (490) |
| 0.006 | 365 | 0.03 | 740 | (303) | |
| 0.08 | >365 | 0.01 | 800 | (496) | |
| 0.23c | 740 | (221, 485b) | |||
| 0.004c–0.025 | 750 | (221, 485b) | |||
| 57 (o-nitrobenzyl, oNB; R = Cl, OH, MeO, NH2, p-MeOC6H4, etc.) | 0.001–0.1 | 325 | 0.015–0.065 | 750 | (190) |
| 265 (3-(4,5-dimethoxy-2-nitrophenyl)-2-butyl, DMNPB) | 0.26 | 365 | 0.17 | 720 | (492) |
| 115 ((4′-methoxy-4-nitrobiphenyl-3-yleth-2-yl)methyl, PMNB) | 0.1 | 315 | 0.45 | 800 | (236) |
| 3.1 | 740 | (237) | |||
| 266 ((4′-tris-ethoxymethoxy-4-nitrobiphenyl-3-yleth-2-yl)methyl, PENB) | 0.1 | 315 | 3.7 | 740 | (493) |
| 117 (2,7-bis-{4-nitro-8-[3-(2-propyl)-styryl]}-9,9-bis-[1-(3,6-dioxaheptyl)]-fluorene, BNSF) | 0.25 | 354 | 5.0 | 800 | (237) |
| 267 ((7-hydroxycoumarin-4-yl)methyl) | 0.025 | 365 | 1.07 | 740 | (331) |
| 0.13 | 800 | (303) | |||
| 268 ((6-chloro-7-hydroxycoumarin-4-yl)methyl) | 0.01 | 365 | 1.07 | 740 | (303) |
| 0.34 | 800 | (303) | |||
| 143 ((6-bromo-7-hydroxycoumarin-4-yl)methyl, BHCM) | 0.084 | 350 | 0.35 | 740 | (288) |
| 0.019–0.037 | 365 | 0.51–1.99 | 740 | (294, 303) | |
| 0.21 | 780 | (330a) | |||
| 0.37–0.42 | 800 | (303) | |||
| 269 ({7-[bis(carboxymethyl)-amino]coumarin-4-yl}methyl, BCMACM) | 0.29 | 330–430 | ca. 1c | 800 | (286) |
| 270 ({7-[4-(dimethylamino)styryl]coumarin-4-yl}methoxycarbonyl) | 0.83 × 10–3 | 407 | 0.26 | 800 | (497) |
| 271 ((3,6,8-tribromo-7-hydroxycoumarin-4-yl)methyl) | 0.065 | 365 | 0.96 | 740 | (303) |
| 3.1 | 800 | (303) | |||
| 181 ((8-bromo-7-hydroxyquinoline-2-yl)methyl, BHQ) | 0.29–0.39 | 365 | 0.59–0.90 | 740 | (331) |
| 0.087 | 780 | (330a) | |||
| 272 ((8-cyano-7-hydroxyquinoline-2-yl)methyl, CyHQ) | 0.31 | 365 | 0.32 | 740 | (335a) |
| 273 ((8-chloro-7-hydroxyquinoline-2-yl)methyl, CHQ) | 0.10 | 365 | 0.12 | 740 | (335a) |
| 274 ([7-(dimethylamino)quinoline-2-yl]methyl, DMAQ) | 0.046 | 365 | 0.13 | 740 | (335a) |
| 275 ([7-(dimethylamino)-4-chloroquinoline-2-yl]methyl, DMAQ-Cl) | 0.09 | 365 | 0.47 | 740 | (335a) |
| 276 ((7-mercaptoquinoline-2-yl)methyl, TQ) | 0.063 | 365 | 0.42 | 740 | (335a) |
| 277 ((Z)-butyl 3-(4-(diethylamino)-2-hydroxyphenyl)-2-acetamidoacrylate) | 0.05 | 300–400 | 0.3 | 750 | (393b) |
| 278 (3,5-dibromo-2,4-dihydroxycinnamate) | 0.05 | 369 | 1.6 | 750 | (390) |
| 0.05 | 300–400 | 0.6 | 750 | (393a) | |
| 279 ((E)-3-(6-hydroxy-benzo(1,3)dioxo-5-yl)acrylate) | 0.03 | 300–400 | 3.8 | 750 | (393a) |
| 280 ((E)-3-(4-diethylamino-2-hydroxy-phenyl)acrylate) | 0.02 | 300–400 | 2.0 | 750 | (393a) |
| 281 ((Z)-butyl 2-acetamido-3-(5-hydroxybenzo[d][1,3]dioxol-6-yl)acrylate) | 0.07 | 300–400 | 2.0 | 750 | (393b) |
| 282 ((E)-3-(8-hydroxy-2,3,6,7-tetrahydro-1H,5H-pyrido(3,2,1-ij)quinolin-9-yl)acrylate) | 0.03 | 300–400 | 4.7 | 750 | (393a) |
| 125 (4-methoxy-7-nitroindolinyl, MNI) | 0.085 | 350 | 0.006 | 730 | (10d, 483) |
| 127 (4-methoxy-5,7-dinitroindolinyl, MDNI) | 0.47 | 350 | 0.06c | 730 | (494) |
| 283 (4-carboxymethoxy-5,7-dinitroindolinyl, CDNI) | 0.5c | 334–364 | 0.06 | 720 | (483a) |
| 130 (5-methoxy-8-nitro-1,2-dihydroquinolinyl, MNDQ) | 0.05 | 365 | <0.06c | 720 | (250) |
The single-photon quantum yield of substrate release.
The two-photon cross section of uncaging.
By comparison with the literature data.
