Table 2. Ru(0)-Catalyzed Arylation of Pyridine Derivativesa.
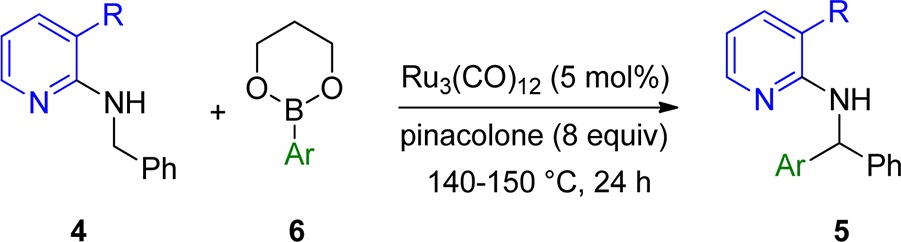
| entry | reactant 4 | R | Ar | product 5 | convb (%) | yieldc (%) |
|---|---|---|---|---|---|---|
| 1 | 4a | Me | Ph | 5a | 86 | 64 |
| 2 | 4a | Me | 2-Me-Ph | 5e | 55 | nid |
| 3 | 4a | Me | 1-naph | 5f | 8 | ni |
| 4 | 4a | Me | 3-Me-Ph | 5g | 87 | 61 |
| 5 | 4a | Me | 3-Cl-Ph | 5h | 59 | 38 |
| 6 | 4a | Me | 4-Me-Ph | 5i | 88 | 62 |
| 7 | 4a | Me | 4-t-Bu-Ph | 5j | 87 | 64 |
| 8 | 4a | Me | 4-OMe-Ph | 5k | 50 | 39 |
| 9 | 4a | Me | 4-F-Ph | 5l | 89 | 66 |
| 10 | 4a | Me | 4-Cl-Ph | 5m | 49 | 33 |
| 11 | 4a | Me | 4-CF3-Ph | 5n | 61 | 41 |
| 12 | 4a | Me | 4-Ac-Ph | 5o | 11 | ni |
| 13 | 4a | Me | 4-NO2-Ph | 5p | 0 | 0 |
| 14 | 4a | Me | 4-CN-Ph | 5q | 0 | 0 |
| 15 | 4a | Me | 3-pyridyl | 5r | 0 | 0 |
| 16 | 4a | Me | 2-thienyl | 5s | 0 | 0 |
| 17 | 4c | CF3 | Phe | 5c | 90 | 78 |
| 18 | 4c | CF3 | 4-Me-Phe | 5t | 92 | 77 |
| 19 | 4c | CF3 | 4-t-Bu-Phe | 5u | 84 | 70 |
| 20 | 4c | CF3 | 4-OMe-Phe | 5v | 76 | 61 |
| 21 | 4c | CF3 | 4-F-Phe | 5w | 65 | 51 |
| 22 | 4d | Ph | Phe | 5d | 100 | 90 |
| 23 | 4d | Ph | 4-Me-Phe | 5x | 100 | 85 |
| 24 | 4d | Ph | 4-t-Bu-Phe | 5y | 100 | 96 |
| 25 | 4d | Ph | 4-F-Phe | 5z | 100 | 72 |
| 26 | 4d | Ph | 4-Cl-Phe | 5aa | 87 | 64 |
| 27 | 4d | Ph | 4-CF3-Phe | 5ab | 65 | 31 |
| 28 | 4d | Ph | 4-Ac-Phe | 5ac | 89 | 52 |
| 29 | 4d | Ph | 4- NO2-Phe | 5ad | 0 | 0 |
| 30 | 4d | Ph | 4-CN-Phe | 5ae | 0 | 0 |
Reaction conditions: 4 (0.5 mmol), 6 (0.75 mmol), Ru3(CO)12 (5 mol %), and pinacolone (0.5 mL).
Conversion based on GC analysis with respect to 4 (dodecane as internal standard).
ni = not isolated.
Could not be isolated because of side products.
150 °C.
