Abstract
The present study examined the effect of melatonin implants on follicle growth in dromedary camels two months ahead of their natural breeding season (December to March). Female camels (n = 6) were treated with melatonin implants at the dose rate of 1 implant per 28 kg body weight sc. Control camels (n = 6) were administered an SC placebo implant of 8 ml vitamin A. Ovarian ultrasonography was performed at weekly interval upto 7 weeks. Camels were mated with virile stud when a follicle (≥10 mm) was visible on either of the ovaries. Blood was collected on day 7, 9, 15, 20, 25 and 30 for assay of plasma progesterone and sonography performed at the same time. Small follicles (2-3 mm) appeared around the periphery of ovaries in 83.3% of camels by day 7 and in 100% camels by day 14. By the end of 7th week an ovulatory size follicle (≥1.0 cm) could be observed in 83.3% of treated camels, and these camels were mated with virile studs. In control group, small follicles appeared at the periphery of ovaries only in 66.6% camels but did not progress in growth except in one camel (16.6%) however, ovulating size (≥10 mm) follicle was not observed in any camel by the end of 7th week. All treated camels ovulated and one treated camel became pregnant while early embryonic death occurred in one camel. Non–pregnant camels of both groups were mated during the breeding season. All camels of treatment group and 33.33% camels of control group became pregnant by the end of breeding season (April 2010). It was concluded that melatonin implants can augment the follicle growth in lactating camels ahead of the breeding season and pregnancy can occur on mating. Fertility of treated camels during the breeding season is improved.
Keywords: Camel, Melatonin implant, Progesterone, Ovary, Ultrasound
Sommario
Nel presente studio viene esaminato l’effetto di impianti di melatonina sulla crescita del follicolo in dromedari - cammelli, nei due mesi precedenti il loro periodo di riproduzione naturale (da dicembre a marzo). Sei femmine di cammello sono state trattate con impianti di melatonina alla dose di 1 impianto per 28 kg di peso corporeo. A sei cammelli (di controllo) è stato somministrato come placebo un impianto di 8 ml di vitamina A per 28 Kg di peso corporeo. L’ecografia è stata eseguita ad intervalli settimanali per 7 settimane. I cammelli sono stati fatti accoppiare quando un follicolo (≥ 10 mm) era visibile in una delle ovaie. Il sangue è stato raccolto al 7°, 9°, 15°, 20°, 25° e 30° giorno, per il dosaggio del progesterone plasmatico e l’ecografia eseguita allo stesso tempo. Follicoli piccoli (2-3 mm) sono apparsi nella periferia delle ovaie nel 83,3% dei cammelli al 7° giorno e nel 100% dei cammelli al 14° giorno. Entro la fine della VII settimana un follicolo ovulatorio di una certa dimensione (≥ 1,0 cm) è stato osservato nel 83,3% dei cammelli trattati, e questi cammelli sono stati fatti accoppiare. Nel gruppo di controllo, sono apparsi piccoli follicoli alla periferia delle ovaie solo nel 66,6% dei cammelli, ma senza progresso nella crescita, fatta eccezione per un cammello (16,6%), tuttavia, la dimensione di un follicolo ovulatorio (≥ 10 mm) non è stata osservata in nessun cammello, entro la fine della VII settimana. Tutti i cammelli trattati hanno avuto un’ovulazione e un cammello è rimasto incinta, mentre è avvenuta la morte precoce di un embrione in un altro cammello. I cammelli non gravidi di entrambi i gruppi sono stati accoppiati durante la stagione riproduttiva. Tutti i cammelli del gruppo di trattamento e 33,33% dei cammelli del gruppo di controllo sono rimasti incinta entro la fine del periodo riproduttivo (aprile 2010).
Si è concluso che gli impianti di melatonina possono aumentare la crescita del follicolo nei cammelli prima della stagione riproduttiva e si può verificare la gravidanza dopo accoppiamento. La fertilità dei cammelli trattati, durante la stagione riproduttiva, è migliorata.
Introduction
During the last few years plenty of work has been carried out in recognizing the effect of photoperiodic cues and melatonin secretion in camel [1–5]. The plasma melatonin levels increase steeply in camel soon after sunset and remain elevated throughout all the night [3], the night concentrations being five times higher than the day time concentrations [5]. The pattern of melatonin secretion shows a seasonal variation parallel to the photoperiodic changes of the year [4,5]. A significant and positive correlation between plasma melatonin and FSH concentration has been recently shown in the Bactrian camel [6]. Thus, the onset of breeding season during longer nights and shorter days usually reported for camels [7–9] can partially be explained by the increasing levels of melatonin stimulating follicle growth and mating during the breeding season [1].
A valid use of the understanding of these physiological rythyms could be phase shifting of reproductive activity in camel by the administration of melatonin implants as has been shown for sheep [10,11], goats [12–14] and mares [15]. Because of a longer gestation period in camels [16] camels conceiving during peak breeding season (March) [8] would parturate at a time when the breeding season is approaching an end. With 30 days required for complete involution [17] camels would be out of the breeding season and would thus conceive during the next breeding season one year later. Thus, attempts to phase shift the breeding season in camel would be advantageous. Trials on female camels by photoperiodic control through application of mask over eyes resulted in follicular activity in a high proportion of females [18] but the approach appears to be less practical as the mask had to be applied for 6 h daily for a period of one to two months. This study examined the effects of commercially available melatonin implants placed subcutaneously in female camels two months ahead of the breeding season on the initiation of follicular growth and the fertility of camels with natural mating.
Materials and methods
Experimental animals
Twelve lactating healthy female camels with no follicular growth (ascertained by ultrasound) belonging to the herd of National research Centre on Camel, Bikaner, India were selected for the present study. Camels were housed in natural daylight during the study period without exposure to any additional light or darkness.
Female camels (n = 6) were treated (1 October 2009) with commercial melatonin implants (Melovine, CEVA, Spain) containing 18 mg of melatonin (at the dose rate of 1 implant per 28 kg body weight subcutaneously) approximately 60 days ahead of the natural breeding season (December to March). An equal number (n = 6) of control camels received a placebo subcutaneous implant of 8 ml of vitamin A (Inj Vetade, Sarabhai Chemicals, India). Both groups were kept under intensive management conditions during the period of study.
Melatonin implants
Commercially available melatonin implants (Melovine, CEVA Salud Animal Barcelona, Spain) containing 18 mg of melatonin were used in the present study. There were no previous reports on the use of melatonin implants in camel, therefore, the dose used for equines (1 implant/28 kg b. wt., s.c.) [15] was used in the present study. The small implants were loaded in a 12 gauze needle and placed subcutaneously under the skin of the neck by pushing the implants with a sterile plunger (Fig. 1).
Figure 1.

The technique of application of subcutaneous melatonin implants in camel.
Ultrasound evaluation of follicular activity and breeding
Ultrasound examination of ovaries and uterus was done using a portable ultrasound machine (AGROSCAN linear, ECM 1"6 BD de la Republique, F 16000 Angouleme, FRANCE) having a dual frequency linear array transducer (5.0–7.5 MHz) to record ovarian activity and folliculogenesis and also for pregnancy diagnosis in she camels. The images were saved using a multimedia kit (Portable Multiple player-100, SIGMATEK) attached to the ultrasound machine. The saved images were subsequently transferred and stored on a computer. The preparation of camels and ultrasound examination was performed as described previously [17]. Briefly, camels were restained by ropes tied to both fore and both hind legs separately in a sternal recumbency. The examinations were done by the examiner sitting close to the animal. The rectum was evacuated of the feces and the probe of the ultrasound covered with a sterile sleeve was introduced in the rectum of the animal. The ovaries and uterine horns were scanned as per the requirement.
The ultrasound examination was done once before the application of melatonin implant and at 7th and 14th day post-implantation followed by weekly examination up to 7th week post-implantation of melatonin implant to monitor the ovarian activity and folliculogenesis in treatment as well as control animals.
The animals, which showed the presence of mature follicle (≥10 mm) were mated with virile stud camels. The mated animals were further examined ultrasonographically to monitor ovarian changes on days 7th, 9th and 15th post-mating and both uterine and ovarian changes for pregnancy diagnosis and ovarian status on days 20, 25, 30, 45 and 60 post-mating.
Serum progesterone assay
Blood samples (10 ml) were collected at 0, 7, 9, 15, 20, 25 and 30 days post-mating for progesterone assay and the serum was separated and stored at −20 °C until subsequent assay for progesterone which was done using commercially available enzyme immuno-assay kits (DIALAB Wiener Neudorf, Austria). The absorbance and progesterone concentration were read on a ELISA reader (Multi Scan M S India) using 450 nm filter. The specificity of the test was high and the intra and inter assay coefficient of variation was 5.7% and 9%, respectively.
Monitoring of reproductive activity during the breeding season
In the present study, the implantation of melatonin implants was done 2 months prior to breeding season in female camels, to augment the folliculogenesis. However, with the onset of breeding season, the camels in which no follicular activity was observed till 7th week post treatment were further examined at weekly intervals to observe the subsequent fertility during the breeding season.
Statistical analysis
The plasma progesterone concentrations in different camels on different days of reproduction and the overall progesterone concentrations were compared by student ‘t’ test.
Result
A total of 114 ultrasonographic examinations were performed on 12 camels to examine ovarian follicular status. The ultrasonographic examination of ovaries in treated camels (n = 6) subsequent to melatonin implants revealed the appearance of follicular growth onset within a week of treatment. This was evident by the appearance of small follicles (2-3 mm) visible as anechogenic area around the periphery of ovaries in 83.3% of treated camels by 7 days and in 100% camels by day 14. A small follicle (≤5 mm diameter) was also evident in one of the ovary in 50% of the treated camels by 7 days post treatment, and in both ovaries in 33.33% of treated camels by the same period (Table 1). The small follicles continued to be visible over the ovaries till clearly demarcated small or large follicles were visible over the ovaries (Fig. 2). A mature ovulatory size (≥10 mm diameter) follicle was visible over either of the ovaries in 16.67%, 33.33%, 16.67% and 16.67% camels by 3rd, 5th, 6th and 7th week post treatment (Table 1, Fig. 2). Overall, by the end of the 7th week post treatment 83.33% of treated camels evidenced a mature follicle, capable of ovulation. In the camel number K 159 a developing small follicle ≥ 5 mm was observed on the left ovary on the 14th day of treatment which attained a size of 12.2 mm in the 3rd week post treatment. The follicular size at the time of mating and subsequent ovulation in camels under treatment ranged from 10.2 to 13 mm. Ovulation occurred in all camels mated to male camels.
Table 1.
Percentage of treated camels (n = 6) showing follicular growth during different weeks post treatment.
| Follicle size | Before treatment | Post treatment |
||||||
|---|---|---|---|---|---|---|---|---|
| 1st week | 2nd week | 3rd week | 4th week | 5th week | 6th week | 7th week | ||
| ≤5 mm | – | 83.34 | 66.67 | 66.67 | 66.67 | – | – | – |
| 5–10 mm | – | – | 16.67 | – | 16.67 | 50.00 | – | – |
| ≥ 10 mm | – | – | – | 16.67 | – | 33.33 | 16.67 | 16.67 |
| Total | – | 83.34 | 83.34 | 83.34 | 83.34 | 83.33 | 16.67 | 16.67 |
| Mating | – | – | – | 16.67 | – | 33.33 | 16.67 | 16.67 |
Figure 2.
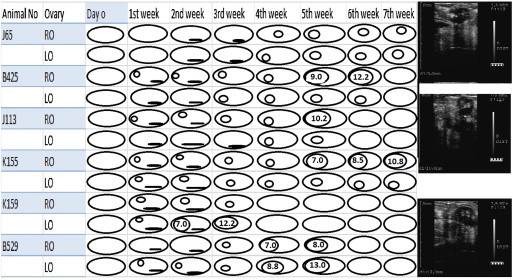
Follicular growth in camels treated with melatonin during 7 weeks post treatment. RO = Right ovary and LO = Left ovary. The black areas represent small follicles (2-3 mm) around the periphery of ovaries whereas the clearly visible individual follicles are shown as round structures. The diameters of follicles are shown. Representative sonograms are shown on the side.
The ultrasonographic examination of untreated camels of the control group revealed no change in the ovarian echotexture by the end of 1st week of the experiment. By the 2nd week, anechoic small follicles visible as a black periphery around the ovaries was detectable over one ovary in 50% camels (K 101, K 455 and J 223) and over both ovaries in 16.6% camels (J 77). The black periphery was visible over both the ovaries by the 5th week of experiment, which continued up to 7th week of treatment. A small follicle (≤5 mm) was observed over the right ovary of 16.6% camels (K 455) by the end of 7th week of experiment. No female camel was observed to have a developing follicle (5–10 mm) or mature (≥10 mm) follicle up to 7th week of the experiment (Fig. 3).
Figure 3.
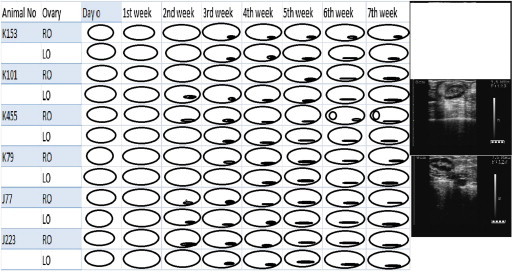
Follicular growths in control camels during the 7 weeks of study. RO = Right ovary and LO = Left ovary. The black areas represent small follicles whereas clearly visible follicles are shown as round structures. Representative sonograms are shown on the side.
Ultrasonographic examination of camels on days 7 and 9 days post-mating revealed the corpus luteum in all camels, reflecting that all mated camels had ovulated. At day 15 post-mating sonographic examination revealed the presence of corpus luteum in two camels only which were considered pregnant. At 20 days however, the CL disappeared in one out of the two camels considered pregnant suggesting an early embryonic death. At days 25, 45 and 60 post-mating the embryonic vesicle, fetus and its parts were visible only in one camel.
The progesterone concentration in camels before mating (day 0) ranged from 0.28 to 0.69 ng/ml. The serum progesterone levels increased to significantly higher (P < 0.05) values of 1.03–1.57 ng/ml at 7th day post-mating in all the mated camels. At 15th day post-mating, progesterone levels were above 1 ng/ml only in two camels J 113 and K 159 (1.51 ng/ml and 1.17 ng/ml), respectively and were considered pregnant. At 20, 25 and 30 days post-mating the progesterone concentrations in camel number J113 declined below 1 ng/ml suggesting an early embryonic death. In camel number K159 the progesterone concentration continued to remain above 1 ng/ml (Table 2). In the other three camels the plasma progesterone concentrations were signinficantly (P < 0.05) lower on day 20, 25 and 30 compared to the plasma concentrations on day 7 and 9. The plasma progesterone concentrations were well correlated with the sonographic findings.
Table 2.
Serum progesterone concentrations in treated camels after mating.
| Camel no. | Progesterone concentration (ng/ml) on days post-mating |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 9 | 15 | 20 | 25 | 30 | |
| B 425 | 0.36 | 1.03 | 1.08 | 0.87 | 0.64 | 0.52 | 0.22 |
| J 13 | 0.32 | 1.57 | 1.35 | 1.51 | 0.82 | 0.56 | 0.51 |
| K 155 | 0.28 | 1.23 | 0.86 | 0.54 | 0.46 | 0.44 | 0.32 |
| K 159 | 0.38 | 1.25 | 1.06 | 1.16 | 1.09 | 1.12 | 1.00 |
| B 529 | 0.69 | 1.08 | 0.77 | 0.95 | 0.74 | 0.55 | 0.57 |
| Mean ± SE | 0.41 ± 0.07 | 1.23 ± 0.09 | 1.03 ± 0.1 | 1.01 ± 0.16 | 0.75 ± 0.24 | 0.63 ± 0.18 | 0.53 ± 0.14 |
During the breeding season (December 2009 to April 2010) mature follicles (<1.0 cm) were observed ultrasonographically in all the camels of control and non-pregnant camels of treatment group. Camels were mated with the stud camels whenever follicles were observed till they conceived and were confirmed pregnant by ultrasonography. All six camels (100%) of treatment group and two (33.33%) camels (J 77, J 223) of control group were found pregnant at the end of breeding season i.e. April 2010.
Discussion
During the present study anechogenic areas of small follicles less than 3 mm were visible in treated and untreated camels along the periphery of the ovaries. These follicles continued to be evident in untreated camels whereas measurable follicles greater than 5 mm appeared in treated camels which continued to grow and reached ovulatory size at different weeks post treatment. Similar presence of small follicles around the periphery of ovaries has been previously described in camels [19] and llamas [20]. Since camels were in the transition period hence recognizable size follicles were absent and small follicles did not progress in growth in untreated camels. In studies on Bactrian camels there were incomplete follicular wave cycles during the transition period completing in 20.3 days whereas the follicular wave cycles were complete during the breeding season completing in 44 days [21]. In the dromedary camel follicle development occurs in a wave fashion charachterised by synchronous growth of a group of follicles followed by continued growth of a dominant follicle and regression of the remaining subordinate follicles [22–24]. The follicular wave patterns vary considerably between camels and can be divided into four phases namely follicular recruitment, the growth phase, the mature phase and the regression phase. During the recruitment phase there is emergence of several follicles (2-3 mm) on the ovarian surface and takes around 2–4 days [25]. There is establishment of one or two dominant follicles during the growth phase of 6–10 days until follicles reach 1.0 cm in diameter [23]. During the mature phase the follicle reaches its maximum diameter and is capable of ovulating and this phase lasts for 7–8 days [23]. These previous findings only partially explain the follicle growth observed during the present study as follicular growth progressed very slowly during the present study requiring up to five weeks in reaching the ovulatory size after their first appearance. Similar to the present study our previous study [18] evidenced the appearance of small follicles by the third week and ovulatory size follicles in 72% of camels by the end of 7th week of application of mask over eyes.
The ovulatory size follicle developed in right ovary in 3 camels and in left ovary in 2 camels. Although the left ovary is known to have more follicles in camel [26–28] the number of camels in the present study is so small that comparison with these would not be appropriate.
The mechanisms of initiation of follicle growth in camels treated with melatonin implants principally involve the alteration in plasma levels of melatonin. It has been shown that the pattern of melatonin secretion in camel varied parallel to the photoperiodic changes [3]. The action of melatonin on folliculogenesis could probably be due to reduction in the prolactin production, as melatonin implants are known to inhibit prolactin secretion [29–31]. Melatonin probably also increases the FSH secretion as a recent study in Bactrian camels showed positive correlations between plasma melatonin and FSH Concentrations [6]. Lactating camels parturated 50–60 days back were used for the present study and suckling is known to inhibit follicle growth in camel [6,17] and weaning and death of the calf is known to initiate follicle growth [26,32].
Camels were mated when follicles greater than 10 mm were visible over the ovaries because previous studies have shown that the optimum time to mate camels is when the follicles are between 10 and 17 mm [8,17,18,32]. All mated camels ovulated as evident by the plasma progesterone and sonographic appearance of a CL on day 7 and 9 post-mating. Previous studies have shown that the plasma concentrations in mated camels are above 1 ng/ml on day 8 of mating but decreased to less than 1 ng/ml on day 10-11 in the absence of pregnancy [22,32,33]. Sonographic findings and plasma progesterone concentrations were well correlated with each other and in the camel in which the CL disappeared after day 15 of mating the plasma progesterone concentrations also fell. A low fertility was obtained by mating melatonin treated camels during the transition period of the breeding season with only one camel becoming pregnant. Previous studies have shown that growth and development of ovarian follicles in camel were greater during the breeding season [8,34] as early breeding season follicles display a low aromatase activity [8].
Melatonin treatment of camels during the transition period also led to improved fertility during the breeding season as 100% of treated camels became pregnant whereas only 33.33% of untreated camels became pregnant by mating during the breeding season. Forcada et al. (2007) [11] had previously recorded from their studies in sheep that melatonin implant treatments had a positive effect on reproductive parameters (including conception) of non-pregnant ewes at the second or third mating during the breeding season.
It was concluded that melatonin implants can augment the follicle growth in lactating camels ahead of the breeding season and pregnancy can occur on mating. Such treatments also result in increased fertility of camels during the breeding season.
Conflict of interest
The authors have no conflict of interests to disclose.
Acknowledgements
The authors thankfully acknowledge Philippe Chemineau, INRA, Chef du Département, “Physiologie Animale et Systèmes d'Elevage” (PHASE), 37380 Nouzilly France for providing the melatonin implants as a gift.
Appendix. Supplementary material
References
- 1.Vyas S., Ravault J.P., Faye B., Chemineau P. The nyctohemeral rhythm of melatonin in camel (Camelus dromedarius) Revue Elev Med Vet Pays Trop. 1997;50:261–263. [Google Scholar]
- 2.El-Allali K., Achaaban M.R., Viven-Roels B., Bothorel B., Tligui N.S., Pevet P. Seasonal variations in the nycthemeral rhythm of plasma melatonin in the camel (Camelus dromedarius) J Pineal Res. 2005;39:121–128. doi: 10.1111/j.1600-079X.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 3.El Allali K., Achaaban M.R., Vivien-Roels B., Bothorel B., Tligui N.S., Pévet P. Seasonal variations in the nycthemeral rhythm of plasma melatonin in the camel (Camelus dromedarius) J Pineal Res. 2006;40:194. doi: 10.1111/j.1600-079X.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdulla A., Al-Qarawi, El-Moughy S.A. Seasonality and the melatonin signal in relation to age as correlated to the sexual cycle of the one humped male camel (Camelus dromedarius) Biol Rhythm Res. 2008;39:131–142. [Google Scholar]
- 5.El-Allali K., Sinitskaya N., Bothorel B., Achaaban R., Pevet P., Simonneaux V. Daily Aa-nat gene expression in the camel (Camelus dromedarius) pineal gland. Chronobiol Int. 2008;25:800–907. doi: 10.1080/07420520802384085. [DOI] [PubMed] [Google Scholar]
- 6.Yong Z. The relationship between melatonin and gonadotropins in the breeding season of camel (Camelus bactrianus) and Yak (Bos grunniens). Ph.D Thesis Gansu Agricultural University Lan Zhou, China2011.
- 7.Agarwal S.P., Khanna N.D., Agarwal V.K., Dwarkanathan P.K. Circulating levels of estrogen and progesterone in female camel (Camelus dromedarius) during pregnancy. Theriogenol. 1987;37:1239–1247. [Google Scholar]
- 8.Sghiri A., Driancourt M. Seasonal effects on fertility and ovarian follicular growth and maturation in camels (Camelus dromedarius) Anim Reprod Sci. 1999;55:223–237. doi: 10.1016/s0378-4320(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 9.Purohit G.N., Pareek P.K. Research on dromedary reproduction: the past two decades and future prospective. Vet Bull. 2000;70:1265–1274. [Google Scholar]
- 10.Malpaux B., Robinson J.E., Brown M.B., Karsch F.J. Importance of changing photoperiod and melatonin secretory pattern in determining the length of the breeding season in the Suffolk ewe. J Reprod Fertil. 1988;83:461–470. doi: 10.1530/jrf.0.0830461. [DOI] [PubMed] [Google Scholar]
- 11.Forcada F., Abecia J.A., Casao A., Cebrián-Pérez J.A., Muiño-Blanco T., Palacín I. Effects of ageing and exogenous melatonin on pituitary responsiveness to GnRH in ewes during anestrus and the reproductive season. Theriogenol. 2007;67:855–862. doi: 10.1016/j.theriogenology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Chemineau P., Normant E., Ravault J.P., Thimonier J. Induction and persistence of pituitary and ovarian activity in the out-of-season lactating dairy goat after a treatment combining a skeleton photoperiod, melatonin and the male effect. J Reprod Fert. 1986;78:497–504. doi: 10.1530/jrf.0.0780497. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S., Purohit G.N. Effect of a single subcutaneous injection of melatonin on oestrous response and conception rate in goats. Small Rumin Res. 2009;82:152–155. [Google Scholar]
- 14.Zarazaga L.A., Gatica M.C., Celi I., Guzmán J.L. Malpaux. Effect of melatonin implants on sexual activity in Mediterranean goat females without separation from males. Theriogenol. 2009;72:910–918. doi: 10.1016/j.theriogenology.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Guillaume D., Arnaud G., Camillo F., Duchamp G., Palmer E. Effect of melatonin implants on reproductive status of mares. Biol Reprod Mon. 1995;1:435–442. [Google Scholar]
- 16.Khanna N.D., Tandon S.N., Rai A.K. Breeding parameters of Indian camels. Indian J Anim Sci. 1990;60:1347–1354. [Google Scholar]
- 17.Vyas S., Sahani M.S. Real time ultrasonography of ovaries and breeding of the one humped camel (Camelus dromedarius) during the early postpartum period. Anim Reprod Sci. 2000;59:179–184. doi: 10.1016/s0378-4320(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 18.Vyas S., Singh R., Purohit G.N., Pareek P.K., Sahani M.S. Ultrasound evaluation of ovarian response to photoperiodic control measures in Camelus dromedarius. Vet Arhiv. 2008;78:39–48. [Google Scholar]
- 19.Tibary A., Anouassi A. Breeding soundness examination of the female camelidae. In: Tibary A., Anouassi A., editors. Theriogenology in camelidae. Abu Dhabi Printing Press; Mina Abu Dhabi: 1997. pp. 243–310. [Google Scholar]
- 20.Adams G.P., Griffin P.G., Ginther O.J. In situ morphologic dynamics of ovaries, uterus and cervix in llamas. Biol Reprod. 1989;41:551–558. doi: 10.1095/biolreprod41.3.551. [DOI] [PubMed] [Google Scholar]
- 21.Nikjou D., Nisari-Nasalji A., Skidmore J.A., Mogheiseh A., Germai A., Razavi K. Ovarian follicular dynamics in Bactrian camel (Camelus bactrianus) J Camel Pract Res. 2009;16:97–105. [Google Scholar]
- 22.Skidmore J.A., Billah M., Allen W.R. Patterns of hormone secretion throughout pregnancy in the one one-humped camel (Camelus dromedarius) Reprod Fertil. 1996;8:863–869. doi: 10.1071/rd9960863. [DOI] [PubMed] [Google Scholar]
- 23.Skidmore J.A. Ovarian kinetics and control of ovulation. In: Skidmore J.A., Adams G.P., editors. Recent advances in camelid reproduction. 2000. http://www.ivis.org [Google Scholar]
- 24.Skidmore J.A. Reproduction in dromedary camels: an update. Anim Reprod. 2005;2:161–171. [Google Scholar]
- 25.Tibary A., Anouassi Ultrasonographic changes of the reproductive tract in the female camel (Camelus dromedarius) during the follicular cycle and pregnancy. J Camel Pract Res. 1996;3:71–90. [Google Scholar]
- 26.El-Wishy A.B. Reproduction in the female dromedary (Camelus dromedarius): a review. Anim Reprod Sci. 1987;15:273–297. [Google Scholar]
- 27.Hussein M.M., El-Garawany A.A., Amin K. Ovarian activity of she camel (Camelus dromedarius) in relation to season, hormonal pattern, age and body condition scores. Beni-Suef Vet Med J. 2008;18:1–9. [Google Scholar]
- 28.El-Harairy M.A., Zeidan A.E.B., Afify A.A., Amer H.A., Amer A.M. Ovarian activity, biochemical changes and histological status of the dromedary she camel as affected by different seasons of the year. Nat Sci. 2010;8:54–65. [Google Scholar]
- 29.Critser J.K., Block T.M., Folkman S., Hauser E.R. Effect of photoperiod on LH, FSH, prolactin and melatonin patterns in ovariectomized prepubertal heifers. J Reprod Fertil. 1987;81:29–39. doi: 10.1530/jrf.0.0810029. [DOI] [PubMed] [Google Scholar]
- 30.Robinson J.J., Wallace J.M., Aitken R.P., Wigzell S. Effect of duration of melatonin treatment on the onset and duration of oestrous cyclicity in ewes. J Reprod Fertil. 1992;95:709–717. doi: 10.1530/jrf.0.0950709. [DOI] [PubMed] [Google Scholar]
- 31.Malpaux B., Daveau A., Maurice F., Locatelli A., Thiéry J.C. Evidence that melatonin binding sites in the pars tuberalis do not mediate the photoperiodic actions of melatonin on LH and prolactin secretion in ewes. J Reprod Fertil. 1994;101:625–632. doi: 10.1530/jrf.0.1010625. [DOI] [PubMed] [Google Scholar]
- 32.Skidmore J.A. Reproductive physiology in female old world camelids. Anim Reprod Sci. 2011;124:148–154. doi: 10.1016/j.anireprosci.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 33.Vyas S., Kishore N., Mal G. Serum progesterone analysis by commercially available EIA kits to monitor ovulation and conception in dromedary camels. J Camel Pract Res. 2010;17:79–83. [Google Scholar]
- 34.Abdoon A.S.S. Factors affecting follicular population, oocyte yield and quality in camels (Camelus dromedarius) ovary with special reference to maturation time in vitro. Anim Reprod Sci. 2001;66:71–79. doi: 10.1016/s0378-4320(01)00078-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


