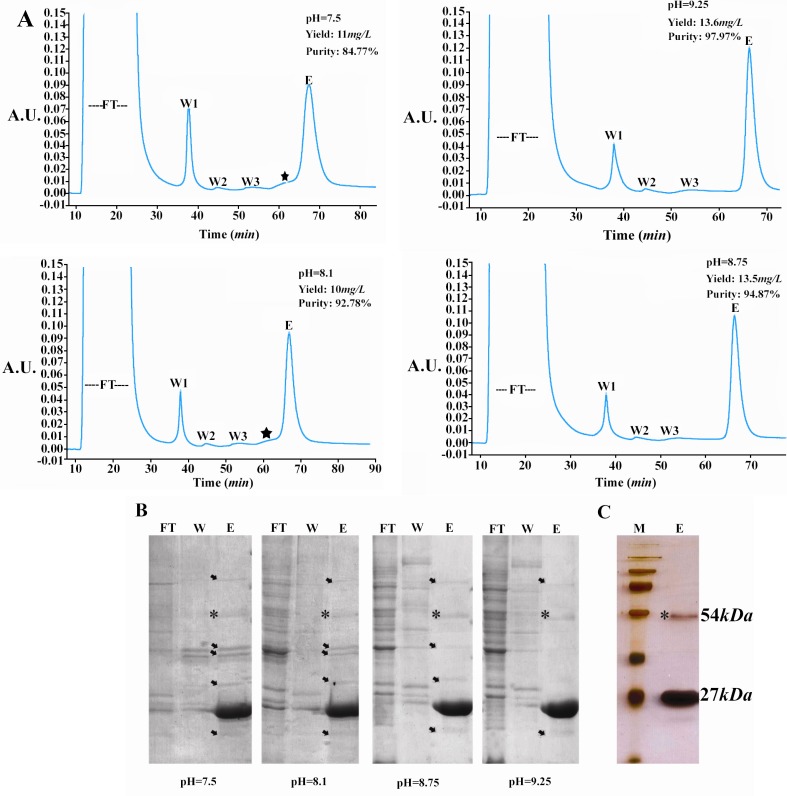
Figure 2.
Purification of TGRA8. A) Chromatograms of the immobilized metal affinity chromatography (IMAC) purification of TGRA8. The purification was performed by using the HisTrap FF column (5 ml) and buffers made at four pH values of 7.5, 8.1, 8.75 and 9.25. Initially, the column was equilibrated with the binding buffer (5 mM imidazole, 50 mM Tris–HCl, 500 mM NaCl). The soluble E.coli lysate was passed though the column and the unbound proteins were collected (Flow Through). The column was then washed step-wised with the binding buffer containing 35 (Wash 1), 60 (Wash 2) and 90 (Wash 3) mM imidazole. Absorbed material was eluted with the binding buffer containing 500 mM imidazole (Elution). The purity and yield of TGRA8 was improved when pH was increased from 7.5 to 9.25. The star mark possibly represents shows the peak of impurities. FT) flow through, W1: wash 1, W2: wash 2, W3: wash 3 and E: Elution. B) SDS-PAGE analysis of TGRA8 purified by IMAC at four different pH. Protein samples of unbound proteins (FT), wash 1 (W) and elution (E) fractions were analyzed. The purity of TGRA8 was considerably improved with increasing the pH from 7.5 to 9.25. The arrows represent bacterial proteins co-purified with TGRA8. The 54 kDa protein band, marked with a star sign, might be the dimmer of TGRA8 as it reacts with Toxoplasma-specific antibody on 12 % SDS-PAGE stained with Coomassie Blue. C) Silver nitrate staining of the purified TGRA8. At pH 9.25 TGRA8 was purified near to homogeneity. On silver stained gel only 2 specific bands of 27 and 54 kDa could be observed. M: Protein molecular weight marker