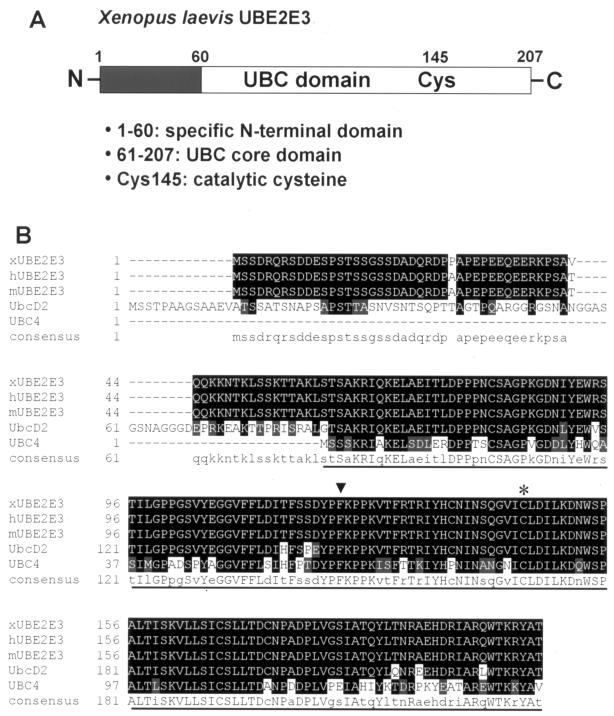
FIG. 1.
X. laevis UBE2E3. (A) Linear view of UBE2E3 showing the N-terminal extension (black box) and the UBC core domain (white) with the catalytic cysteine (Cys145). The numbers are amino acid positions. (B) Amino acid sequence alignment of the primary sequences of X. laevis UBE2E3 (xUBE2E3) and its orthologues in human (hUBE2E3), mouse (mUBE2E3), D. melanogaster (UbcD2), and yeast (UBC4). The highly conserved UBC core domain is underlined. The catalytic cysteine at position 145 (asterisk) and the phenylalanine at position 122 (arrowhead), the key residue for the interaction with HECT E3s, are indicated. Conserved amino acids in the different species are indicated by white letters on black background and are shown as capital letters in the consensus sequence. Conservative changes in the different species are indicated by white letters on gray shaded background and are shown as lowercase letters in the consensus sequence. Gaps introduced to maximize sequence alignment are indicated by dashes.