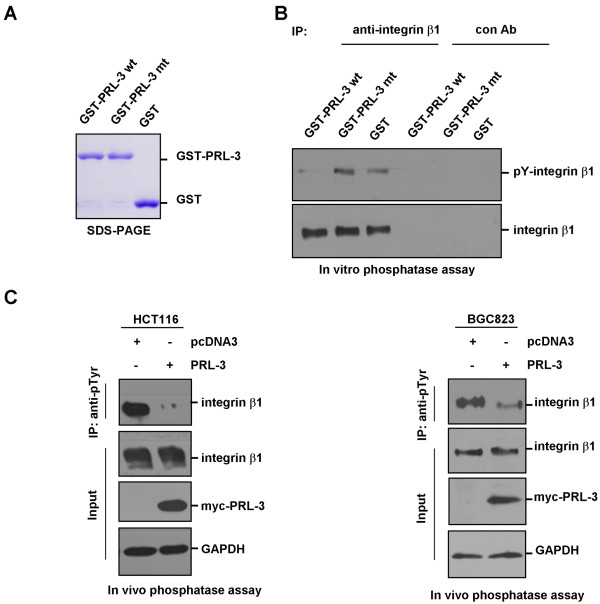
Figure 3.
In vitro and in vivo dephosphorylation of integrin β1 by PRL-3. (A) The purified GST-PRL-3, mutant GST-PRL-3 (GST-PRL-3-mt) and GST proteins were examined by SDS-PAGE and commassie blue staining. (B) Dephosphorylation of integrin β1 by PRL-3 in vitro. 1000 μg HCT116 cell lysates were immunoprecipitated with integrin β1 antibody or control IgG. After sequential washing with lysis buffer and dephosphorylation buffer, the precipitates were used as substrates, and incubated with 1 μg purified GST-PRL-3 wt, GST-PRL-3 mt or GST in the dephosphorylation buffer for 30 min at 30°C, then the mixtures were analyzed by Western blotting with anti-phosphotyrosine (4G10) and anti-integrin β1 antibodies. (C) Dephosphorylation of integrin β1 in HCT116 (left panel) and BGC823 (right panel) cells by ectopic expression of PRL-3. Equal amount of lysates from cells over-expressing myc-PRL-3 and pcDNA3 (control) were immunoprecipitated with anti-phosphotyrosine (4G10) antibody, followed by immunoblotting with anti-integrin β1 antibody. Expression of integrin β1 and myc-PRL-3 in the lysates were indicated.