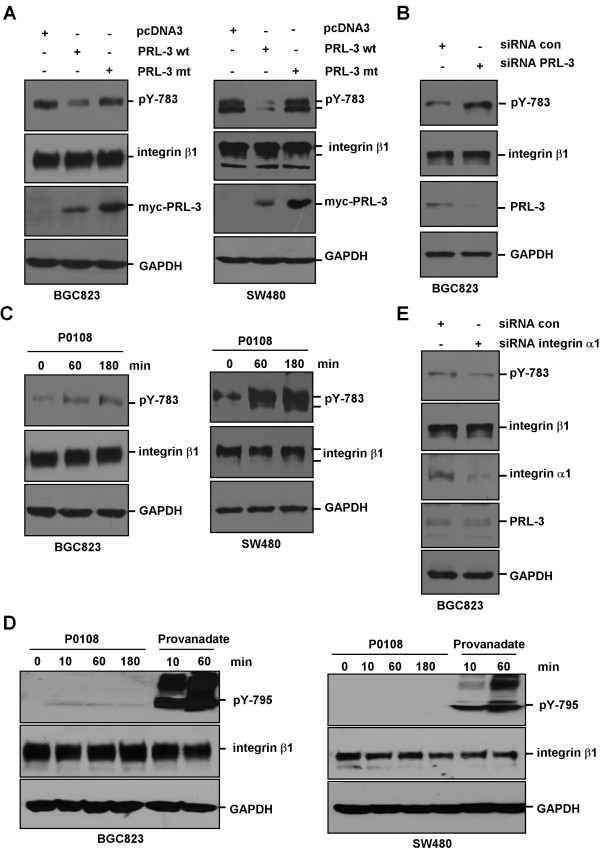
Figure 4.
Regulation of integrin β1 phosphorylation at tyrosine 783 by PRL-3. (A) Wild-type myc-PRL-3, but not mutant myc-PRL-3 dephosphorylated tyrosine 783 of integrin β1 in BGC-823 (left panel) and SW480 cells (right panel). After transfection with myc-PRL-3, myc-PRL-3-mt or pcDNA3 (control) plasmids, equal amount of cell lysates were analyzed by Western blotting with antibodies against pY783, integrin β1, myc and GAPDH. (B) Knockdown of PRL-3 promoted pY783 phosphorylation. BGC823 cells were transfected with 100 pmol indicated siRNA for 48 hours, and the cell lysates were analyzed by Western blotting with indicated antibodies. (C) PRL-3 inhibitor enhanced pY783 phosphorylation. BGC823 (left panel) and SW480 cells (right panel) were treated with 10 μg/ml PRL-3 inhibitor. Cells were harvested at different time points and protein lysates were analyzed by Western blotting with indicated antibodies. (D) PRL-3 had minimal effect on pY795 of integrin β1. BGC823 (left panel) and SW480 cells (right panel) were treated with 10 μg/ml PRL-3 inhibitor or 100 μM provanadate (as positive control). Protein lysates were harvested at indicated time points and analyzed by Western blotting. (E) Knockdown of integrin α1 decreased pY783 phosphorylation. BGC823 cells were transfected with integrin α1-specific siRNA or control siRNA for 72 hours, and the lysates were analyzed by Western blotting.