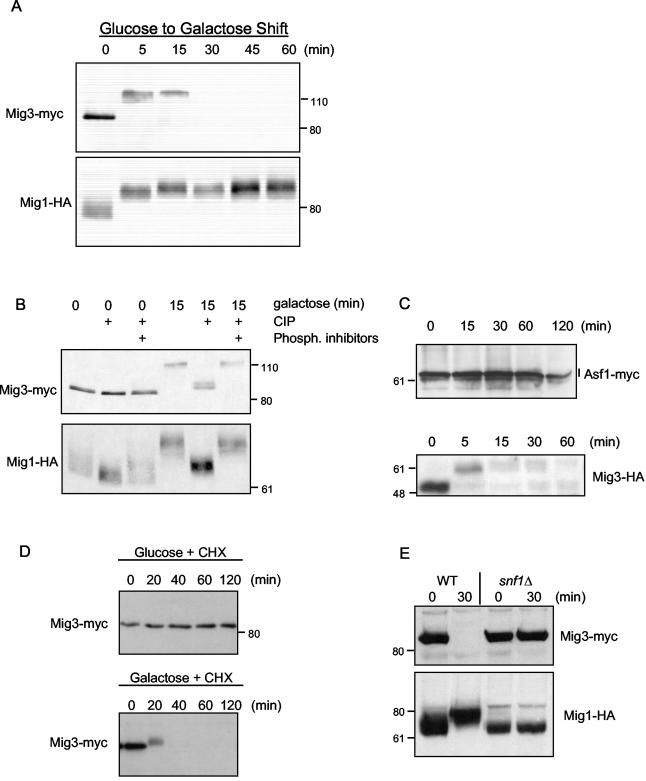
FIG. 1.
Mig3 is phosphorylated and degraded after shifting cells from glucose to galactose in an Snf1-dependent manner. (A) Mig3-Myc cells (CDY61) with a centromeric plasmid expressing Mig1-HA (48) were cultivated in a synthetic complete medium containing 2% glucose and lacking uracil. Cells were then washed once and resuspended in a synthetic complete medium containing 2% galactose and lacking uracil for the periods of time indicated in the figure. Proteins were extracted from TCA-precipitated cells, and Mig3-Myc and Mig1-HA levels as well as electrophoretic mobility were monitored by immunoblotting. (B) Protein extracts prepared from the 0- and 15-min galactose shifts described above were treated with or without 10 U of CIP. Phosphatase inhibitors were added to some reactions as a control showing that the mobility shifts were due to the action of the CIP. (C) Asf1-13Myc cells (CMY1202) and Mig3-HA cells (CMY1274) were shifted from glucose to galactose medium for the indicated periods of time, and proteins were extracted and analyzed by immunoblotting. (D) Mig3-Myc (CDY55) cells in YPD were split in two. One-half was treated with 50 μg of cycloheximide (CHX) per ml, whereas the other half was washed and resuspended in the same volume of YP plus 2% galactose plus 50 μg of CHX per ml; Mig3-Myc protein levels were evaluated by immunoblotting. (E) Mig3-Myc and Mig1-HA levels as well as electrophoretic mobility were monitored in wild-type (CDY61) and snf1Δ cells (CDY75) at 0 and 15 min after a glucose-to-galactose shift. The positions of molecular weight markers are shown next to the immunoblots.