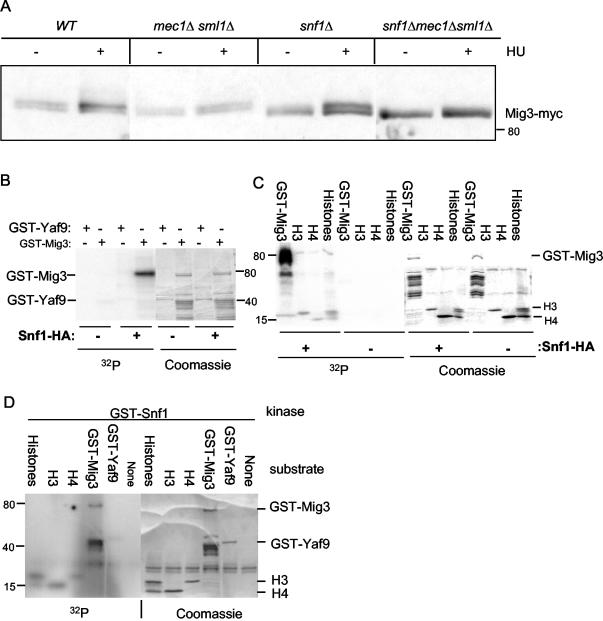
FIG. 3.
Mig3 phosphorylation in vivo after genotoxic stress is dependent on both Mec1 and Snf1 kinases, and Mig3 is an in vitro substrate for the Snf1 kinase. (A) Immunoblot analysis of Mig3 in vivo phosphorylation before or after a 2-h treatment with 200 mM HU was done with the wild-type (WT) (CDY61), mec1Δ sml1Δ (CDY205), snf1Δ (CDY75), or snf1Δ mec1Δ sml1Δ (CDY212) strains expressing Mig3-Myc. Panels B through D show that Snf1 phosphorylates GST-Mig3 in vitro. (B and C) The Snf1-HA kinase was immunoprecipitated from protein extracts prepared from SNF1-HA yeast cells. A control immunoprecipitation was done on protein extracts from the wild-type untagged strain. The left-hand panels show PhosphorImager scans of SDS-polyacrylamide gels showing [32P]phosphate incorporation into GST-Mig3 and histones H3 and H4 but not into a control GST-Yaf9 fusion protein. The right-hand panels show the Coomassie blue-stained gels. (D) The GST-Snf1 kinase purified from E. coli weakly phosphorylates GST-Mig3 and the histones H3 and H4 in vitro but not the GST-Yaf9 protein. The left-hand panel shows a PhosphorImager scan of the kinase reaction products after SDS-PAGE, and the right-hand panel shows the Coomassie blue staining of the same gel.