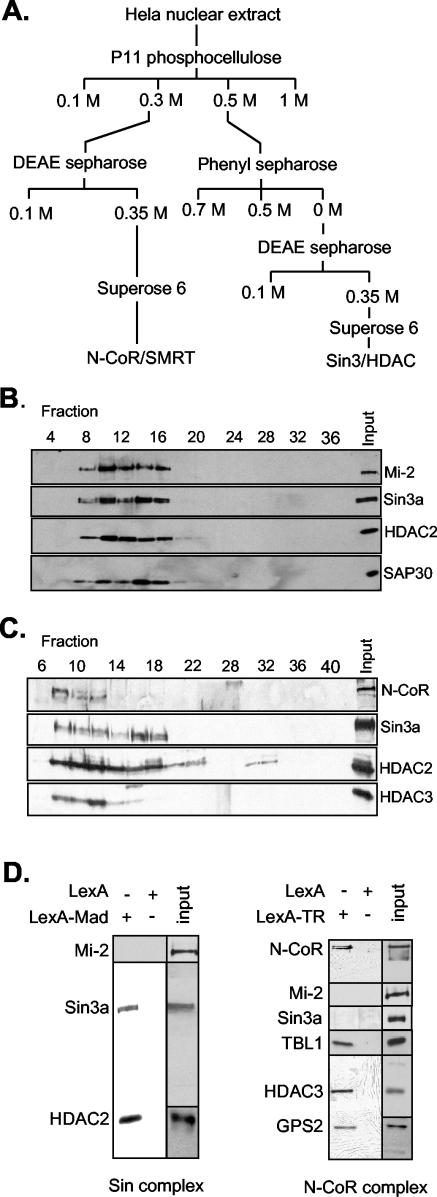
FIG.1.
Purification of the Sin3/HDAC and N-CoR/SMRT complexes and recruitment to DNA by using chimeric repressor molecules. (A) Conventional purification of the Sin3/HDAC and N-CoR/SMRT complexes. HeLa cell nuclear extract was fractionated as shown. The Sin3/HDAC and N-CoR/SMRT complexes were monitored throughout the purification by Western analysis with antibodies against HDAC2, mSin3a, SAP30, N-CoR, and HDAC3. (B) Elution profile of the Sin3/HDAC complex on a Superose 6 column analyzed by Western blotting against Sin3a, HDAC2, and SAP30. (C) As in panel B, using the N-CoR/SMRT fractions and antibodies against N-CoR, HDAC2, HDAC3, and Sin3a. (D) Recruitment of the Sin3/HDAC and N-CoR/SMRT complexes by the LexA-Mad and LexA-TR(DE) fusion proteins, respectively. Fractions 9 to 13 (Fig. 1B) and 7 to 11 (Fig. 1C) from the Superose 6 columns containing the Sin3/HDAC and N-CoR/SMRT complexes were used for the LexA-Mad and LexA-TR(DE) pull-down on paramagnetic streptavidin-conjugated Dynal Dynabeads containing multimerized LexA oligonucleotides. Bound proteins were eluted from DNA with 1 M NaCl, loaded on a sodium dodecyl sulfate-polyacrylamide (8%) gel, and analyzed by Western blotting with the indicated antibodies. Input fractions used for the pull-down are also shown.