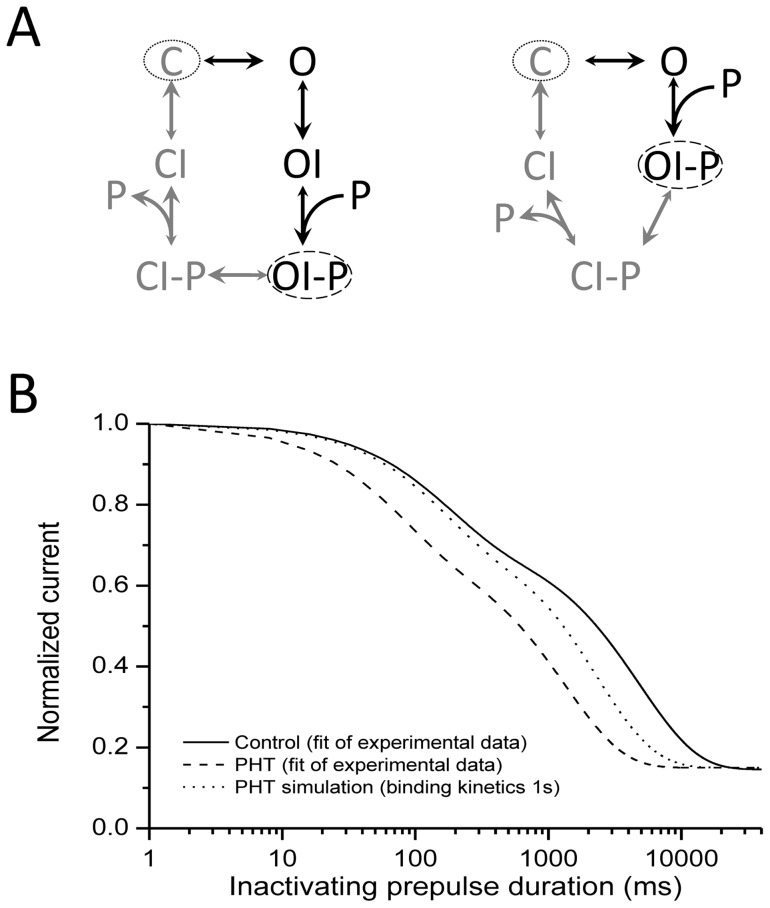
Figure 10. Inferences on the mechanism of action.
A; simplified gating schemes illustrating the action of PHT, excluding an interaction with the open state of the channel. The scheme on the left depicts PHT as a pure inactivated state stabilizer that binds to the inactivated state of the channel; the scheme on the right is an interpretation of our results and depicts PHT as an inactivated state stabilizer that binds to the inactivated state and to a hypothetical intermediate in the inactivation pathway. C, O, OI and CI are the closed, open, open-inactivated and closed-inactivated states of the Na+ channel; P is PHT, which in both cases has much higher affinity to the I state than to the C (resting) state. The black parts of the schemes are the transitions induced by depolarized potentials: the dimension of the arrowhead indicates the value of the rate constants and the dashed oval indicates the absorbing state at depolarized potentials. The gray parts are the transitions induced by repolarizations and the dotted oval is the absorbing state in these conditions. The main difference between the two schemes is the fact that on the left PHT binds to channels already in the inactivated state stabilizing it, whereas on the right it can also accelerate the kinetics of inactivation by binding to a hypothetical intermediate, similarly to a catalyst in a chemical reaction. B; the simulated curve of development of INaP inactivation shows that the effect of PHT cannot be obtained with a simple intrinsic slow binding of PHT to inactivated channels as in scheme A, left. The dotted line is a simple simulation of the maximal effect of PHT on development of INaP inactivation at +40 mV in Layer V neurons according to the scheme in A, left. It is assumed that that the action of PHT is intrinsically slow, developing with a time constant of 1 s (Kuo and Bean, 1994; Kuo et al, 1997), and that it binds irreversibly to inactivated channels. Solid and dashed lines are the fits of the experimental data in control and with PHT respectively, which are shown in Figure 4 and in Table 1. The simulated curve (hDt, development of inactivation in the presence of PHT) has been obtained with the following equation: hDt = ht{1-[(1-ht) dt]}, in which ht is the curve of development in control (fraction of channels available as a function of time at +40 mV) and dt the fractional binding of PHT (kinetics of PHT binding). It is evident that the simulated curve does not approximate the experimental curve in the presence of PHT (compare the dashed line with the dotted one). In order to quantitatively compare the parameters with those of the experimental curves (Table 1), the simulated curve was fit with a double exponential relationship that gave the following parameters: τ1 = 0.12 s, A1 = 0.25, τ2 = 2.4 s, A2 = 0.6, baseline = 0.15, which are different in comparison with those obtained from the experimental curve in the presence of PHT (Table 1). Thus, PHT effect cannot be simulated with a simple intrinsic slow binding of PHT to the channels in the inactivated conformation; an acceleration of the rate constants of development of INaP inactivation as in scheme A, right, is more consistent with the experimental data (see text).