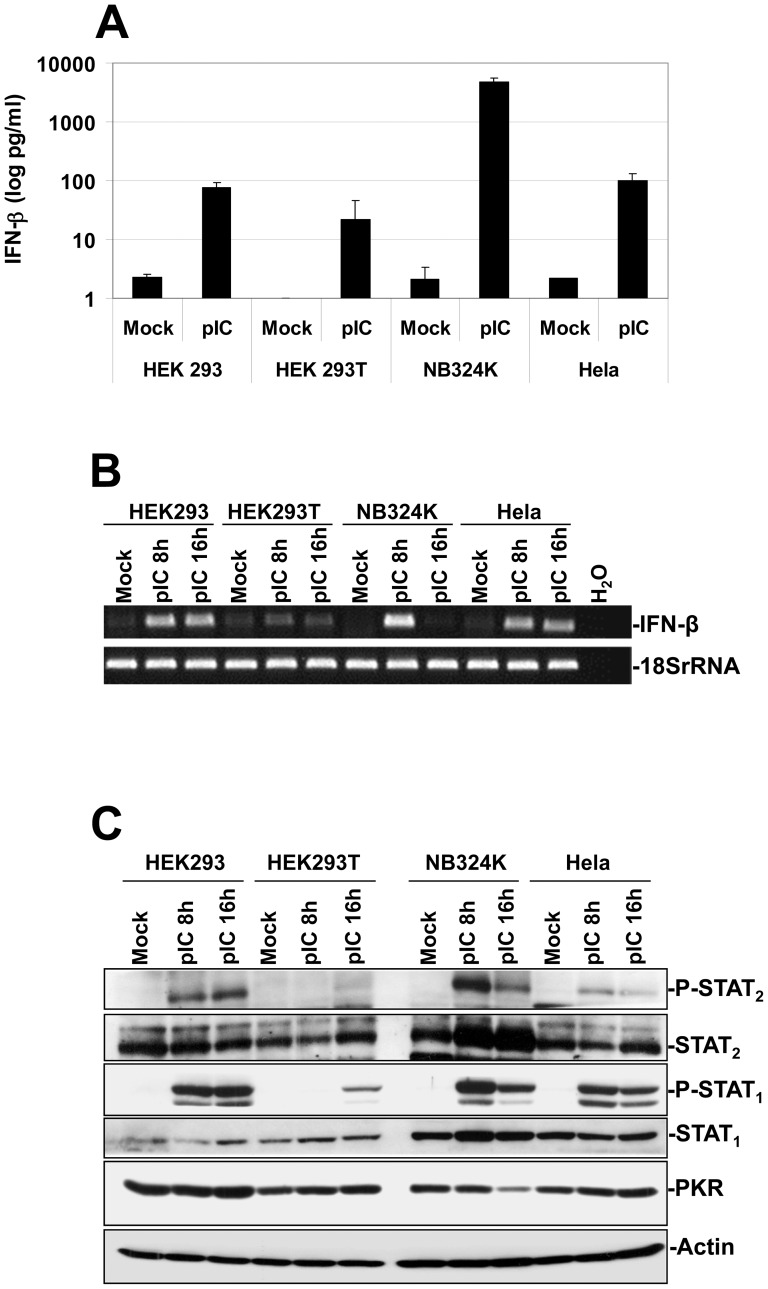
Figure 1. Stimulation of an IFN-mediated antiviral response in human transformed or tumor cells upon Poly(I:C) transfection.
(A) HEK293, HEK293T, NB324K and Hela cultures were transfected with 2 µg/ml of synthetic dsRNA Poly(I:C) (pI:C) or a 150 mM NaCl solution as control, using lipofectamine 2000 for the period indicated in the figure. The respective culture media were then collected, centrifuged to discard cellular debris, and analyzed by Enzyme-linked Immuno-Sorbent Assay (ELISA) for their content in human IFN-β. Each result is represented as mean+standard deviation of three independent experiments. (B) Expression of IFN-α and IFN-β transcripts in mock-treated or pI:C-transfected human cell lines was assessed by RT-PCRs. At the indicated time points, total RNAs were extracted from the respective cultures using the RNeasy kit. One µg of RNA was then treated by DNase I in order to remove potential contaminating DNA and reverse transcribed into cDNA. A fraction of the obtained cDNA (1/10) was then analyzed by PCR for its content in type-I IFN transcripts using specific primer pairs. Transcripts encoding the Human 18S ribosomal protein were used as internal loading controls. Data shown are representative of 3 experiments which gave similar results. (C) Assessment of the IFN-signaling (Jak/STAT) pathway activation in HEK293, HEK293T, NB324K and Hela cells upon pI:C transfection. At the time indicated mock-treated or pI:C-transfected (2 µg/ml) cultures were harvested by scraping in PBS and centrifuged. Cell pellets were then re-suspended in complete Ripa buffer supplemented with phosphatase and protease inhibitors. Total proteins were extracted from each sample as described in Materials and Methods. Fifty µg total proteins per sample were then subjected to bipartite 8/10% SDS-PAGE, transferred onto membranes, and probed with antibodies specific for phosphorylated and total isoforms of STAT1 and STAT2 as well as with an antibody specific to PKR. Actin was used as an internal loading control. Each presented blot is representative of 3 additional which gave similar results.