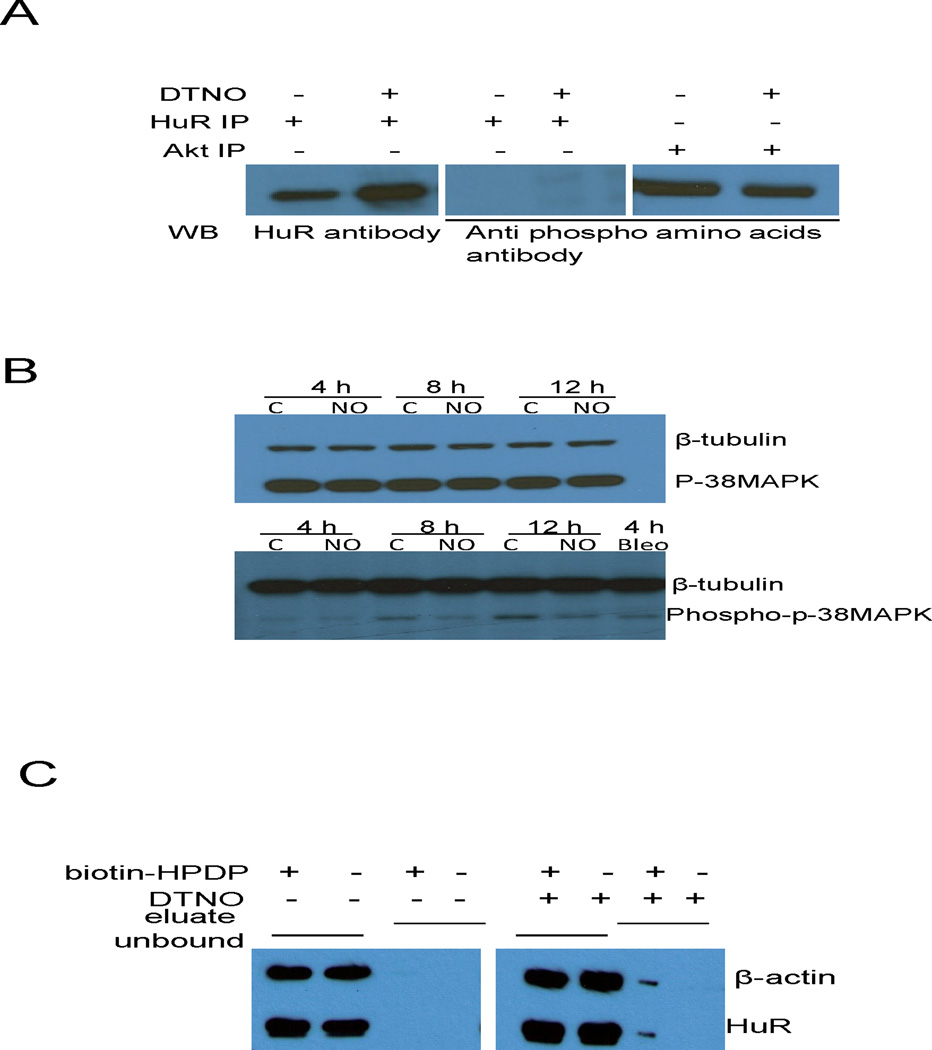
FIGURE 7.
S-nitrosylation of HuR by NO reduces its binding to DAF mRNA. (A) Ishikawa cells were exposed to DTNO (1 mM) for 4 h and the HuR protein was immunoprecipitated. Phosphorylation status of HuR in the IP sample was examined by western blot using anti-phosphoaminoacids antibody. Akt, which is constitutively phosphorylated in Ishikawa cells, was used as positive control. (B) Ishikawa cells were exposed to DTNO (1 mM) for 4, 8 and 12 h, and the expression of p38 MAP kinase and phospho-p38 MAP kinase was assessed by western blot. Bleomycin exposed cells were used as positive control. (C) Ishikawa cells were exposed to DTNO (1 mM) for 4 h, S-nitrosylation of HuR was examined by biotin switch assay. The eluate fraction contains neutravidin pull-down biotinylated proteins in the cell lysates including S-nitrosylated proteins that were biotinylated in the presence of biotin-HPDP. The unbound fraction contains non-biotinylated proteins existing in the supernatant of neutravidin pull-down. Presence of HuR in both the fractions was analyzed by western blot. The β-actin was used as positive control for S-nitrosylated protein.