Figure 2.
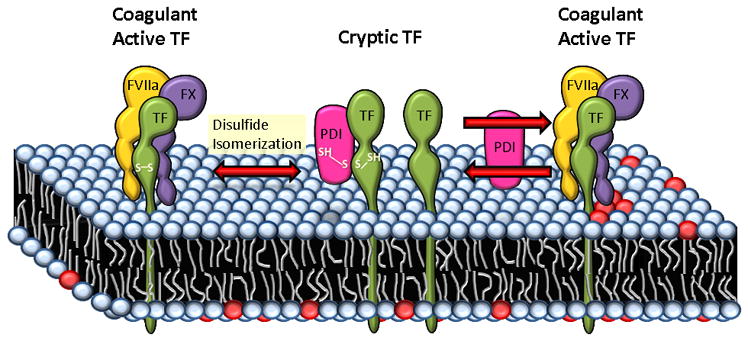
Proposed mechanisms of PDI-mediated regulation of TF coagulant activity at the cell surface. As per one of the proposed models, Cys186-Cys209 disulfide bond of TF is critical for its coagulant activity and post-translational modifications of this allosteric disuflide bond such as reduction, S-nitrosylation and glutathionation keep TF in a cryptic state. PDI can regulate the coagulant activity of TF through formation/breaking or reshuffling of this disulfide bond by its oxido-reductase or isomerase activity. PDI could also regulate TF coagulant activity through S-nitrosylation and/or glutathionation of thiols in TF. In a second model, PDI through its oxido-reductase activity modulates PS dynamics at the cell surface. PDI reductase activity helps in maintaining a low exposure of PS at the cell surface by affecting both flippase and floppase activities. Inhibition of PDI at the cell surface increases PS (shown as red color circles) exposure, which facilitates the conversion of cryptic TF to the coagulant active form. Both PDI-mediated disulfide bond switch and PDI-induced changes in the lipid environment may occur simultaneously and their net effect may dictate TF activity status at the cell surface.
