Abstract
Central airways stenosis (CAS) after lung transplant is a poorly understood complication. Objectives of this study were to determine if CAS was associated with chronic rejection or worse survival after transplant as well as to identify factors associated with CAS in a large cohort of lung transplant recipients. Lung transplant recipients transplanted at a single center were retrospectively reviewed for the development of CAS requiring airway dilation. 467 subjects met inclusion criteria with 60 (13%) of these developing CAS requiring intervention. Of these 60 recipients, 22 (37%) had resolution of CAS with bronchoplasty alone, while 32 (53%) ultimately required stent placement. CAS that required intervention was not a risk factor for the development of bronchiolitis obliterans syndrome or worse overall survival. Significant risk factors for the subsequent development of CAS in a time-dependant multivariable model were pulmonary fungal infections and the need for post-operative tracheostomy. While CAS was not associated with BOS or worse survival, it remains an important complication after lung transplant with potentially preventable risk factors.
Keywords: lung transplantation, stenosis, stent, infection
Introduction
Lung transplantation is a viable option for advanced lung disease with increasing numbers of transplants performed each year. Post-operative care of lung transplant recipients may be complicated by obstruction of the main or lobar bronchi, a condition known as central airway stenosis (CAS). CAS may include the anastomosis but importantly is distinguished from early transplant literature that focused on severe anastomotic stenosis which frequently caused fatal airways obstruction (1). As surgical technique improved, catastrophic anastomotic failure became a rare phenomenon (2) While occurrence is limited in some centers, main and lobar stenosis continues to be a concern, with some programs reporting up to 15% of patients developing CAS post lung transplantation (3–6).
The implications of CAS as well as risk factors leading to CAS are not well defined given the overall low incidence rates, with many previous reports based on small cohorts (3, 7–10). The objective of the present study was to understand the prognostic significance of CAS as well as identify risk factors for the subsequent development of CAS in a large cohort of lung transplant recipients. As prior observations by our group as well as others have shown links between infections and the development of BOS (11, 12), we hypothesized that there may be strong associations between airway infections and CAS development.
Materials and Methods
Study Cohort
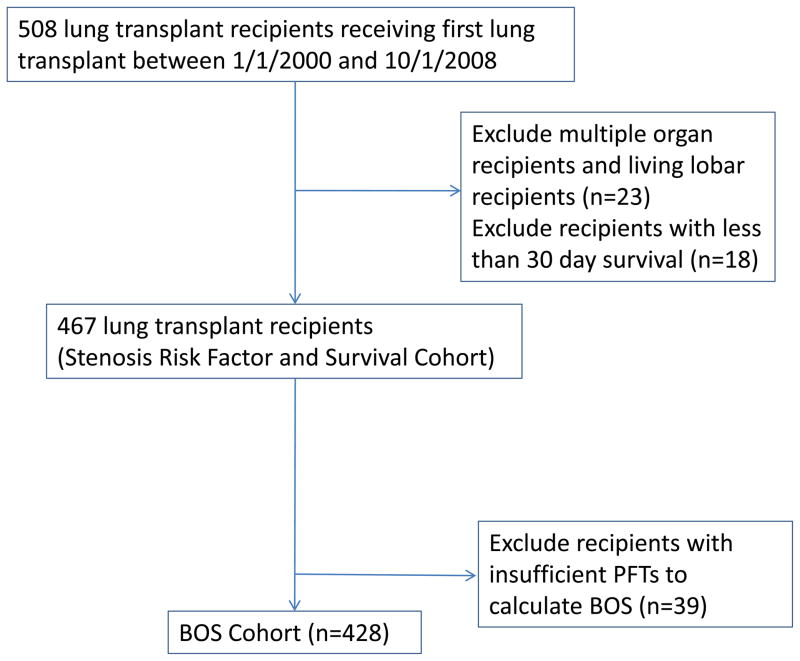
Lung transplant recipients transplanted at Duke University Medical Center between January 1, 2000 and October 1, 2008 were eligible for analysis. Exclusion criteria included: multi-organ transplant, re-transplant and living lobar transplant recipients and survival less than 30 days. Variables for analysis were abstracted from retrospective chart review. The study protocol was approved by the Duke University IRB (protocol #00013461).
Procurement and surgical technique
Standard lung procurement and implantation techniques for our center have been previously described(13). All of the organs were flushed with an extracellular preservation solution with antegrade and retrograde flushing during procurement. All patients included in this study underwent bilateral sequential lung transplantation with an institutional bias towards the bilateral transverse sternothoracotomy (i.e. Clamshell incision). The donor bronchus is transected 1 to 2 cartilaginous rings proximal to the first bifurcation bilaterally and care is taken to preserve peri-bronchial soft tissue during the dissection. The anastomosis is created with a 4–0 absorbable monofilament suture in a running fashion. The membranous portion of the airways appose end-to-end, while a subtle telescoping occurs anteriorly along the cartilaginous edge. No attempt to revascularize bronchial arteries is performed and no tissue coverage of the airway anastomosis occurs. The majority of transplants were performed by 2 surgeons, with 2 others performing a small number of procedures. Three of the surgeons were trained by the senior surgeon, and so technique is homogeneous within the group.
Pre- and Post-transplant clinical protocol
All recipients had uniform induction immunosuppression with tacrolimus, azathioprine, and an IL-2 receptor antagonist at the time of transplantation as previously reported (13). Maintenance immunosuppression included prednisone, tacrolimus and azathioprine. No changes in immunosuppression or surgical technique occurred during the study interval. Fungal prophylaxis included inhaled amphotericin B daily for 4 days immediately post-transplantation followed by weekly administration until time of discharge. In addition, daily nystatin oral rinses were given for at least 6 months post-transplant. All pathogenic bacterial, fungal, viral and mycobacterial isolates were treated. No changes in pathogen treatment were made based on the presence of CAS.
Surveillance bronchoscopy with transbronchial biopsies was performed at 1, 3, 6, 9 and 12 months post-transplant and annually thereafter, or as clinically indicated. Clinical indications include decline in spirometry (either home or office based spirometry) and/or the development of respiratory symptoms.
Acute rejection definition
Acute rejection was defined and graded according to the International Society for Heart and Lung Transplantation recommendations (14). Acute rejection ratio is the sum of the rejection grades divided by the number of evaluable biopsies to normalize the differing numbers of biopsies between subjects (12).
Chronic allograft dysfunction definition
BOS was defined by a progressive decline in spirometry in accordance with guidelines from the International Society for Heart and Lung Transplantation (15). In order to be eligible in our analysis for BOS, recipients had to survive 180 days and have at least 6 pulmonary function tests.
Early allograft dysfunction after lung transplant
Variables considered as indicators for allograft dysfunction included: postoperative tracheostomy, length of hospital stay after transplant, use of extracorporeal membrane circulation (intraoperative and postoperative), and primary graft dysfunction (PGD) grade 3 at 72 hours post reperfusion as defined by the International Society for Heart and Lung Transplantation guidelines..
Allograft infection definition
Allograft infections were determined from bronchoalveolar lavage and bronchial biopsy cultures and included all pathogenic organisms. As a risk factor for CAS, infections were considered up to the point of CAS identification. Infections at the time of CAS identification were also noted.
CAS Definition and Treatment
Airway stenosis was defined as the inability to pass a 6.3 mm diameter bronchoscope through a normally traversable airway. CAS onset date was retrospectively confirmed by chart review of bronchoscopy procedure notes. Initial suspicion of CAS was made by a transplant pulmonologist with confirmation of CAS done by either a thoracic surgeon (prior to 2004) or one of two interventional pulmonologists (2004 and after). All physicians were in agreement as to the definition of CAS.
A stenosis was considered resolved when it was traversable during bronchoscopy without need for further intervention at the site during the study follow-up period. Bronchoscopy notes and acquired images were reviewed to determine if exudative plaques involving the anastomotic site were present at the time of identification of stenosis and first intervention. For the purposes of this analysis, only the time to the first bronchoplasty was considered. A sensitivity analysis considered number of bronchoplasties and number of stenotic airway sites. Our center’s treatment algorithm for CAS consisted of 1–3 serial bronchoplasties 3 to 5 weeks apart or as clinically indicated, followed by stent placement if CAS persisted.
Spirometric Changes
Spirometry results were reviewed for each subject with CAS to assess for change associated with the development of CAS. Baseline forced expiratory volume in the first second (FEV1) values were taken within 30 days prior to the last bronchoscopy that did not reveal evidence of CAS. At least 2 test results with FEV1 values within 10% of each other had to be available for review to establish a baseline FEV1. The post-CAS FEV1 was taken within one month following CAS identification, and prior to any airway intervention. A significant decline in spirometry was defined as a decrease in FEV1 of 200ml. For analysis of changes in spirometry related to intervention, the baseline FEV1 was the last measurement taken within 30 days prior to the initial intervention. The post-intervention FEV1was defined as the first measurement taken after the final recorded intervention.
Statistical Analysis
Study cohort demographics were determined within each group and compared using Chi-square test, Fisher’s exact test or Wilcoxon test as appropriate. Survival and freedom from BOS were analyzed using Kaplan-Meier models. Proportional hazards assumptions were satisfied. Bronchoplasty was considered as a time-dependent event as well as a fixed time independent event in survival analyses. Bronchoplasty was considered as a time-dependent event in the BOS analysis. To analyze the risk factors for CAS requiring bronchoplasty, univariate regression analysis of time dependent and time independent predictors were used to predict the time dependent outcome of CAS requiring bronchoplasty. Predictors significant at the 0.05 significance level were then incorporated into a multivariable model.
Results
Demographics of Study Cohort
508 lung recipients received a first lung transplant at Duke University Medical Center between January 1, 2000 and October 1, 2008. Of these, 23 multi-organ and living lobar recipients and 18 recipients with less than 30-day survival were excluded leaving a study cohort of 467 recipients (Figure 1). Median follow-up time was 3.73 years (IQR 2.15, 5.66). Sixty (13%) subjects developed CAS requiring bronchoplasty after transplant. Within the CAS group, the median number of bronchoplasties was 2 with the majority occurring at a single anatomic location. The first bronchoplasty occurred in the first 6 months of transplant in 72% and virtually all had their first bronchoplasty within the first year after transplant (93%).
Figure 1.
Cohort diagram
The study cohort and group demographics are outlined in Table 1. Comparing recipients with and without CAS requiring intervention, there was no difference in gender, race, native lung disease, type of transplant (single/bilateral), age at transplant, or ischemic time. There were significant differences in variables suggesting initial allograft dysfunction, including longer hospital length of stay immediately after transplant (p=0.006) and a higher rate of tracheostomy post-transplant (p=0.0001) in the CAS group. However, there was no significant difference in primary graft dysfunction (grade 3) or use of ECMO intraoperatively or postoperatively between the groups.
Table 1.
Demographics; Cohort = 467total patients
| No dilation procedures (n=407) | Dilation procedure (n=60) | P value | |
|---|---|---|---|
|
| |||
| Gender, male, n (%) | 226(56) | 37 (62) | 0.37 |
|
| |||
| Race, n (%) | 0.09 | ||
| Caucasian | 361(89) | 54 (90) | |
| Black | 43 (11) | 4 (7) | |
| Other | 3 (0.01) | 2 (3) | |
|
| |||
| Native Lung Disease, n (%) | 0.19 | ||
| Obstructive | 167 (41) | 27 (45) | |
| Vascular | 11 (3) | 1 (2) | |
| Cystic | 78 (19) | 5 (8) | |
| Restrictive | 151(37) | 27 (45) | |
|
| |||
| Type of Transplant, bilateral, n (%) | 393 (97) | 59 (100) | 0.18 |
|
| |||
| Age at transplant, median (IQR) | 56 (43, 62) | 57 (50,62) | 0.14 |
|
| |||
| Ischemic time in minutes, median (IQR) | 366 (311, 445) | 389 (323, 432) | 0.56 |
|
| |||
| Length of stay posttransplant, median (IQR) | 13 (9, 23) | 18 (12, 32) | 0.003 |
|
| |||
| ECMO intraop and postop, n (%) | 17 (0.05) | 3 (0.05) | 1.00 |
|
| |||
| Tracheostomy posttransplant, n(%) | 28 (6.9) | 13 (22) | 0.0002 |
|
| |||
| Developed BOS (based on 428subjects eligible) | 150 (37) | 25 (46) | 0.72 |
|
| |||
| Number of PFTs, median (IQR) | 28 (20, 38) | 29 (19.5, 37.5) | 0.71 |
|
| |||
| Follow-up period, median days (IQR) | 1353 (748, 2190) | 1418 (822, 1717) | 0.28 |
CAS Discovery, Location, and Management
Median time to first bronchoplasty was 140 days (IQR 103, 186) after transplant. There was a relatively short time frame from last bronchoscopy without CAS to first bronchoscopy noting CAS (median of 42.5 days (IQR 28, 62)). Of the 60 subjects with CAS, twenty seven subjects (45%) had symptomatic shortness of breath at the time of bronchoscopy leading to the CAS identification, while another 27 (45%) had no clinical symptoms but CAS was identified during surveillance bronchoscopy. The indication for bronchoscopy could not be determined for the remaining 6 subjects.
The most common site of CAS requiring dilation was the bronchus intermedius, followed by the left and right mainstem (Table 2). Most sites were treated with 2 to 3 bronchoplasties prior to stenting or resolution of CAS with the exception of the bronchus intermedius which was treated with an average of 4 bronchoplasties. Subjects with resolution of CAS using bronchoplasty alone required 1 to 2 procedures, although one patient had 9 interventions prior to the resolution of the stenosis. There was not an association between location of CAS and need for stent placement. Twenty-eight patients (47%) were managed with bronchoplasty alone. Of these 28 recipients that only underwent bronchoplasty, 22 had resolution of CAS on follow-up bronchoscopy, 5 had residual CAS that was thought to be either unstentable or not clinically significant and 1 was lost to follow-up. Thirty-two subjects (53%) with CAS ultimately required airway stenting, with a total of 54 stents placed. CAS was associated with exudative plaques at the site of stenosis in 20 (33%) of patients. The distribution of stenotic sites was similar in patients with or without plaques present at the diagnosis of CAS.
Table 2.
Location of and Number of Stenoses and Dilations and CAS Managed with Dilation Alone
| All Recipients with CAS (N=60) | CAS Managed with Dilation Alone (N=28) | |||
|---|---|---|---|---|
| Location | Stenosis | Dilation | Stenosis | Dilation |
| R Mainstem | 20 | 53 | 6 | 10 |
| R Upper Lobe | 9 | 18 | 2 | 2 |
| Bronchus Int. | 31 | 124 | 10 | 25 |
| R Middle Lobe | 7 | 14 | 3 | 4 |
| R Lower Lobe | 1 | 4 | 0 | 0 |
| L Mainstem | 22 | 60 | 10 | 14 |
| L Upper Lobe | 11 | 30 | 7 | 12 |
| L Lower Lobe | 5 | 11 | 2 | 2 |
R = right, Bronchus Int.= bronchus intermedius, L= left
CAS Effects on FEV1
Spirometry with adequate baseline values before and after initial identification of CAS was available for 45 subjects (75%). Of those available for analysis, 24 subjects (40%) had significant decline (at least 200mL) in spirometry associated with the development of CAS with median FEV1 loss of −0.44L (IQR −0.65L, −0.36L), while 21 subjects (35%) did not have significant change in spirometry with a median FEV1 reduction of −0.02L (IQR −0.1L, 0.09L).
Spirometric data taken within one month prior to the first intervention and following the final intervention was available for 43 subjects (72%) with CAS. Of those, 21 underwent stent placement, while 22 subjects were treated with bronchoplasty alone. Among subjects who received a stent, median FEV1 just prior to initial intervention was 1.84L (IQR 1.30, 2.02) with median increase in FEV1 by 0.38L (p < 0.0207, IQR 0.07, 0.65) after the final intervention. In subjects with CAS treated with bronchoplasty alone, median initial FEV1 was 1.79L (IQR 1.58, 2.44) with median increase in FEV1 of 0.12L (p < 0.0280, IQR −0.09, 0.42).
CAS requiring dilation is not associated with worse survival
In the survival analysis, bronchoplasty was first considered as a time independent variable occurring in the first 6 and 12 months (conditional on 6 or 12 month survival respectively) after transplant. Bronchoplasty was not a predictor for survival in either model. In a Cox regression model, bronchoplasty as a time dependent variable was not a predictor for survival (HR 1.44, 95% CI 0.93, 2.22).
CAS requiring bronchoplasty is not associated with BOS
428 subjects were eligible for BOS analysis having survived at least 180 days and having 6 pulmonary function tests after transplant. There was no difference in median number of PFTs per subject in each group. Median follow-up in this subset was 4.03 years (IQR 2.73, 5.97 years). Similar percentages in each group developed BOS. In the CAS group, 18 of the 25 that developed BOS had bronchoplasty previously with almost all of these occurring over a year after bronchoplasty. Bronchoplasty as a time-dependent variable was not associated with the development of BOS (HR 1.26, 95% CI 0.77 – 2.05).
CAS Risk Factors include fungal infection and postoperative tracheostomy
To define the risk factors for CAS, bronchoplasty was considered as a time dependent outcome with both time dependent and time independent predictors. Time independent predictors included length of stay after transplant operation, post-operative tracheostomy, and ischemic time (ischemia data available for 387 subjects). The time dependent variables of acute rejection ratio as well as the first fungal, bacterial, viral and mycobacterial infections were considered before bronchoplasty. Of note, 2 viral infection, 6 bacterial infection and 8 fungal infections diagnosed at the time of CAS identification. In almost all of the initial allograft infections, the source was bronchoalveolar lavage samples with only 3 positive culture results derived from lung biopsy specimens. Airway infections were identified a median of 52 days (IQR 42,75) prior to initial bronchoplasty in subjects with CAS. We performed an analysis for collinearity among infectious organisms and did not find a significant association.
In univariate analyses, length of stay, post-operative tracheostomy and time to first fungal infection were associated with bronchoplasty and included in the multivariable model. In the multivariable model, only post-operative tracheostomy (p < 0.002) and time to first fungal infection before bronchoplasty (p < 0.0083) were significant predictors for bronchoplasty (Table 3).
Table 3.
Risk factors for CAS requiring dilation after transplant N=467
| Univariate analysis | |||
|---|---|---|---|
| HR | 95% CI | P value | |
| Length of stay | 1.01 | 1.01–1.02 | 0.0006 |
| Postoperative tracheostomy | 3.67 | 1.98–6.79 | <0.0001 |
| Ischemic time (n=387) | 1.00 | 0.99–1.00 | 0.64 |
| Acute rejection ratio * | 1.09 | 0.71–1.67 | 0.69 |
| Fungal infection * | 2.12 | 1.27–3.55 | 0.004 |
| Bacterial infection * | 1.70 | 0.96–3.01 | 0.07 |
| Mycobacterial infection * | 1.20 | 0.72–2.00 | 0.48 |
| Viral infection * | 1.62 | 0.94–2.79 | 0.08 |
| Multivariable analysis | |||
|---|---|---|---|
| HR | 95% CI | P value | |
| Length of stay | 1.01 | 1.00–1.01 | 0.09 |
| Postoperative tracheostomy | 2.92 | 1.47–5.80 | 0.002 |
| Fungal infection * | 2.01 | 1.20–3.37 | 0.0083 |
Time dependent predictor and considered only before dilation
Time dependent predictor and considered only before dilation
Discussion
Although CAS is the most commonly recognized airway complication after lung transplantation, many reports focused just on stenosis at the anastomosis and have been performed in small cohorts (2, 7, 9, 10, 16). This report is the largest cohort analysis performed to date of CAS at both anastomotic sites and lobar airways requiring bronchoplasty after lung transplant. While the incidence of and location of CAS confirms prior studies, the size of the cohort and extended follow-up allow for additional insight into CAS.
An important finding of our study is that the development of CAS and subsequent management with bronchoplasty does not increase the risk of BOS or affect survival after transplant. Although we had relatively few interventions among the total population, the finding is robust with an 80% power (Type-I error rate = 0.05 (two-tailed)) to detect a relative risk of 1.45 of BOS and CAS, and 1.41 of survival and CAS respectively. Our conclusion suggests that the mechanisms underlying central and peripheral airways obstruction are likely different. This finding also indicates that management of CAS with serial bronchoplasties followed by stent placement if necessary, is not associated with subsequent BOS development.
The etiology of CAS is poorly understood. Airway ischemia is thought to play a significant role in the development of CAS due to the dual nature of the bronchial circulation and the common practice of not performing revascularization of the bronchial arteries at the time of transplantation. Although animal models demonstrate the development of collateral circulation approximately 14–21 days following transplantation (17, 18), a recent study of human lung transplant recipients noted decreased airway oxygen saturation in all lobes of the transplanted lung compared to the native lung up to one year post-transplant (19). We found that the majority of CAS develops in the first year, suggesting that ischemia may influence the development of airway stenosis. However, almost half of the identified stenoses resolved or stabilized with bronchoplasty alone, indicating there may be other transient, or reversible influences that also drives CAS.
In our analysis of clinical risk factors that precede the development of CAS, we identified two factors associated with subsequent CAS development. First, pulmonary fungal infections were strongly associated with the development of CAS. Given this finding, we carefully evaluated all fungal cultures from both groups. Considering first fungal infections before CAS, we found that Aspergillus species was the most common organism, representing 55% and 56% of the total fungi identified in the non-CAS and CAS groups respectively. Previous reports have described Aspergillus related anastomotic CAS, often presenting early in the post-transplant period, and associated with ischemic necrosis of the airway mucosa (3, 4, 6, 8, 10, 20). It is unclear from these reports whether Aspergillus is causative of the subsequent CAS or simply opportunistic on the necrotic tissue. In the present study, anastomotic plaques had already resolved, or were not present in 66% of subjects with CAS at the time of first intervention, suggesting fungal associated CAS occurs independent of the presence of anastomotic plaques at the time of diagnosis. This may be an important distinction for future studies examining optimal CAS management strategies.
Bacterial and mycobacterial infections were also noted to have increased incidence in our population with CAS. However, these associations were not significant when modeled as time dependent variables. Most other studies did not perform time dependent analysis which may explain some of the inconsistent associations between infection and airway complications. For example, Moreno et al. noted an association between CAS and Cytomegalovirus infection (4), while Weder et al. and Choong et al. found no such association (6, 8). Similarly, we did not find that bacterial infections were associated with CAS as noted in other reports (4, 8, 10). Unlike most prior studies, we treated infections as a time dependent variable, which minimized the confounding effect of the stenosis or stenosis management as a risk factor for infection.
However, censoring the CAS group at time of bronchoplasty resulted in a shorter period for infection to develop, as well as eliminating our ability to detect infections acquired after bronchoplasty. This conservative analysis potentially biased us towards finding more infections in the non-CAS cohort and reduced our sensitivity to finding additional infectious associations with CAS. Additionally, we have not accounted for multiple repeated cultures or clearance of a specific organism. Thus, our analysis does not answer the question of whether clearance of fungal infections is associated with resolution of CAS.
In addition to fungal infections, we found that the length of hospital stay and need for tracheostomy in the early post-transplant period was associated with the development of CAS in univariate analyses. In the multivariable model, only tracheostomy status was associated with the development of CAS requiring bronchoplasty. While this would imply allograft dysfunction, there was no difference in PGD grade 3 at 72 hours. Several other groups have shown increases in overall airway complications, including CAS, associated with prolonged ventilation (4, 7, 8), ICU stay (7, 8) or hospitalization (4). There are several possible explanations for our finding. Our center has adopted a relatively early approach to tracheostomy after lung transplant. Thus, recipients may have a tracheostomy placed for multiple reasons that may not relate to allograft dysfunction. A prospective approach to data collection with careful attention to perioperative factors and allograft dysfunction may clarify if initial allograft dysfunction is associated with subsequent CAS.
Management of CAS is controversial, and without a widely accepted approach among centers. Our center has defined CAS as the inability to traverse a normally traversable airway with a large (6.3mm OD) flexible bronchoscope, recognizing that larger airways will undergo a proportionally greater decrement in diameter than will more distal airways. We have based this decision on clinical observations that even large airways reduced to a 7 or 8mm diameter rarely cause symptoms or airway collapse and so the diameter of the bronchoscope was selected as the criteria for intervention. Our practice has been to perform serial bronchoplasty up to 3 times followed by stent insertion if the stenosis size or location is amenable to stenting. Using this approach we have been able to resolve CAS in 22 (37%) of patients with balloon dilation alone which is comparable to other centers, though a wide range of success has been reported (5, 16, 21). Both bronchoplasty and stent placement appear to improve declines in FEV1 associated with CAS, although greater improvements in FEV1 were associated with stenting. This is may be due to the fact that larger airways tend to be treated with stents (Table 2) and so are more likely to obstruct greater volumes of lung distal to the stenotic site. Our series included a large number of stenoses involving sites distal to the surgical anastomoses, which is a less commonly encountered presentation of CAS. It is not clear if the mechanisms causing anastomotic CAS also result in distal airways CAS. However, we do note that groups of airways may be either simultaneously, or sequentially stenotic, (e.g. the right mainstem, bronchus intermedius, and right middle lobe) suggesting the primary insult is the same for all sites, and the development of CAS at these sites is part of the evolution of abnormal wound healing within these airways.
Our center has been aggressive in treating fungal infections with both inhaled and systemic therapies (13,22). Given our findings of strong associations between fungal infections and airway stenosis, it may be possible that intensified anti-fungal prophylaxis could reduce the incidence of CAS. In addition, bronchoplasty in combination with anti-fungal therapy may be effective for treating CAS, avoiding the need for stent placement. Appropriate prospective studies are needed to better understand prevention and management of CAS.
In conclusion, we have analyzed a large single center cohort for CAS requiring bronchoplasty and carefully detailed the risk factors for the development of CAS post-lung transplantation. Using a time-dependent analysis, we show strong associations between both fungal infections and post-operative tracheostomy and the subsequent development of CAS. Importantly, our current management of CAS does not appear to affect overall survival or the development of BOS. Future studies are needed to consider underlying mechanisms and optimize treatment of CAS with special attention to fungal infection and allograft dysfunction as key mediators of CAS formation.
Acknowledgments
Funding sources: This work was supported by NIH K24-091140-01 (SMP), American Society of Transplantation Clinical Faculty Development Award (LS).
List of abbreviations
- BOS
bronchiolitis obliterans syndrome
- CAS
central airways stenosisp
- ECMO
extracorporeal membrane oxygenation
- FEV1
forced expiratory volume in 1 second
- IQR
interquartile range
- PFT
pulmonary function testing
- PGD
primary graft dysfunction
Footnotes
Conflicts of interest: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Shennib H, Masard G. Airway complications in lung transplantation. Ann Thorac Surg. 1994;57:506–11. doi: 10.1016/0003-4975(94)91038-3. [DOI] [PubMed] [Google Scholar]
- 2.Santacruz J, Mehta A. Airway complications and management after lung transplantation. Proc Am Thorac Soc. 2009;6:79–93. doi: 10.1513/pats.200808-094GO. [DOI] [PubMed] [Google Scholar]
- 3.Herrera J, McNeil K, Higgins R, Coulden R, Flower C, Nashef S, et al. Airway complications after lung transplantation: treatment and long-term outcome. Ann Thorac Surg. 2001;71:989–94. doi: 10.1016/s0003-4975(00)02127-5. [DOI] [PubMed] [Google Scholar]
- 4.Moreno P, Alvarez A, Algar F, Cano J, Espinosa D, Cerezo F, et al. Incidence, management and clinical outcomes of patients with airway complications following lung transplantation. Eur J Cardio-Thoracic Surg. 2008;34:1198–205. doi: 10.1016/j.ejcts.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Murthy S, Blackstone E, Gildea T, Gonzalez-Stawinski G, Feng J, Budev M, et al. Impact of anastomotic airway complications after lung transplantation. Ann Thorac Surg. 2007;84:401–9. doi: 10.1016/j.athoracsur.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Weder W, Inci I, Korom S, Kestenholz P, Hillinger S, Eich C, et al. Airway complications after lung transplantation: risk factors, prevention and outcome. Eur J Cardio-Thoracic Surg. 2009;35:293–8. doi: 10.1016/j.ejcts.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez A, Algar J, Santos F, Lama R, Aranda J, Baamonde C, et al. Airway complications after lung transplantation: a review of 151 anastomoses. Eur J Cardio-Thoracic Surg. 2001;19:381–87. doi: 10.1016/s1010-7940(01)00619-4. [DOI] [PubMed] [Google Scholar]
- 8.Choong C, Sweet S, Zoole J, Guthrie T, Mendeloff E, Haddad F, et al. Bronchial airway anastomotic complications after pediatric lung transplantation: Incidence, cause, management, and outcome. J Thorac Cardiovasc Surg. 2006;131:198–203. doi: 10.1016/j.jtcvs.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa T, Iacono A, Orons P, Yousem S. Segmental nonanastomotic bronchial stenosis after lung transplantation. Ann Thorac Surg. 2000;69:1020–24. doi: 10.1016/s0003-4975(99)01556-8. [DOI] [PubMed] [Google Scholar]
- 10.Thistlethwaite P, Yung G, Kemp A, Osbourne S, Jamieson S, Channick C, et al. Airway stenoses after lung transplantation: Incidence, management, and outcome. J Thorac Cardiovasc Surg. 2008;136(6):1569–75. doi: 10.1016/j.jtcvs.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Garantziotis S, Howell D, McAdams H, Davis R, Hensaw N, Palmer S. Influenza pneumonia in lung transplant recipients clinical features and association with bronchiolitis obliterans syndrome. Chest. 2001;119:1277–80. doi: 10.1378/chest.119.4.1277. [DOI] [PubMed] [Google Scholar]
- 12.Snyder L, Finlen-Copeland C, Turbyfill W, Howell D, Willner D, Palmer S. Cytomegalovirus pneumonitis is a risk factor for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care. 2010;181(12):1391–6. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartwig M, Snyder L, Finlen-Copeland A, Lin S, Zaas D, Davis R, et al. Lung Transplantation at Duke University. Clin Transpl. 2009:197–210. [PubMed] [Google Scholar]
- 14.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. Journal of Heart & Lung Transplantation. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 15.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. Journal of Heart & Lung Transplantation. 2002;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 16.DeGracia J, Culebras M, Alvarez A, Catalan E, DeLaRosa D, Maestre J, et al. Bronchoscopic balloon dilation in the management of bronchial stenosis following lung transplantation. Respiratory Med. 2007;101:27–33. doi: 10.1016/j.rmed.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Siegelman S, Hagstrom J, Koerner S, Veith F. Resoration of bronchial arterial circulation after canine lung allo-transplantation. J Thorac Cardiovasc Surg. 1977;73:792–5. [PubMed] [Google Scholar]
- 18.Pearson F, Goldberg M, Stone R, Colapinto R. Bronchial arterial circulation restored after reimplantation of canine lung. Can J Surg. 1970;13:243–50. [PubMed] [Google Scholar]
- 19.Dhillon G, Zamora M, Roos J, Sheahan D, Sista R, Starre PVd, et al. Lung transplant airway hypoxia a diathesis to fibrosis? Am J Respir Crit Care Med. 2010;182:230–6. doi: 10.1164/rccm.200910-1573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nunley D, Gal A, Vega D, perlino C, Smith P, Lawrence E. Saprophytic fungal infections and complications involving the bronchial anastomosis following human lung transplantation. Chest. 2002;122:1185–91. doi: 10.1378/chest.122.4.1185. [DOI] [PubMed] [Google Scholar]
- 21.Chhajed P, Malouf M, Tamm M, Spratt P, Glanville A. Interventional bronchoscopy for the management of airway complications following lung transplantation. Chest. 2001;120:1894–9. doi: 10.1378/chest.120.6.1894. [DOI] [PubMed] [Google Scholar]
- 22.Palmer S, Drew R, Whitehouse J, Tapson V, Davis R, McConnell R, et al. Safety of aerosolized amphotericin B lipid complex in lung transplant recipients. Transplantation. 2001;72(3):545–8. doi: 10.1097/00007890-200108150-00036. [DOI] [PubMed] [Google Scholar]