Figure 2.
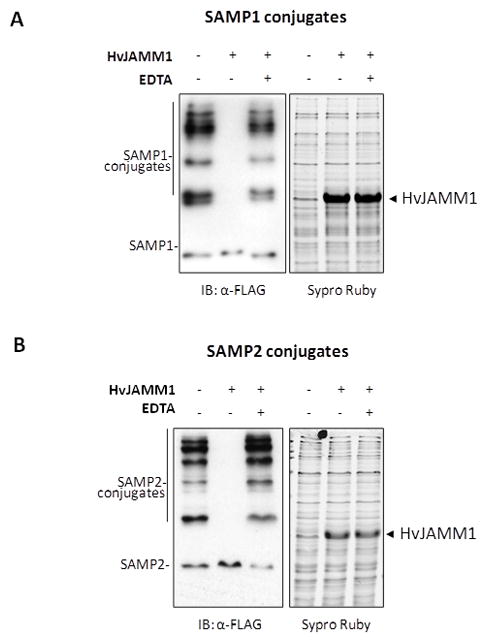
HvJAMM1 is a Zn2+-dependent JAMM metalloprotease. Desampylation activity of HvJAMM1 was monitored using Flag-SAMP1 S85K (A) and Flag-SAMP2 (B) conjugates as described in Experimental Procedures. Similar results were observed for Flag-SAMP1 conjugates. EDTA was included as a negative control to disrupt HvJAMM1 activity based on the conservation of residues in the HvJAMM1 amino acid sequence that are likely to coordinate a catalytic zinc ion. Total protein was stained with Sypro Ruby to confirm equal loading and qualitatively assess whether or not HvJAMM1 is a non-specific protease that hydrolyzes the cellular proteome.
