Figure 6.
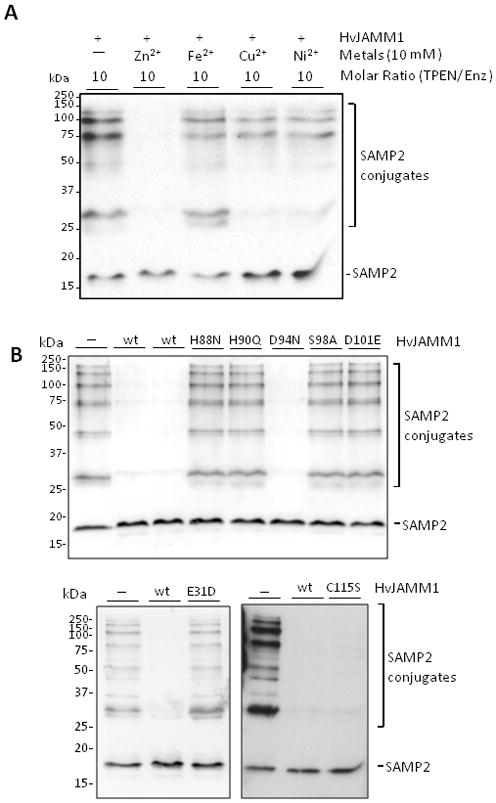
Divalent Zn2+ (A) and conserved active site residues (E31, H88, H90, S98 and D101) (B) are crucial for HvJAMM1 activity. In contrast, HvJAMM1 C115S and D94N variants retain desampylating activity. Reactions were performed using SAMP2 conjugates as a substrate under standard assay conditions (as described in Experimental Procedures). The metal chelator TPEN, divalent metals and HvJAMM1 wild-type and site-directed variant proteins were included in assays as indicated.
