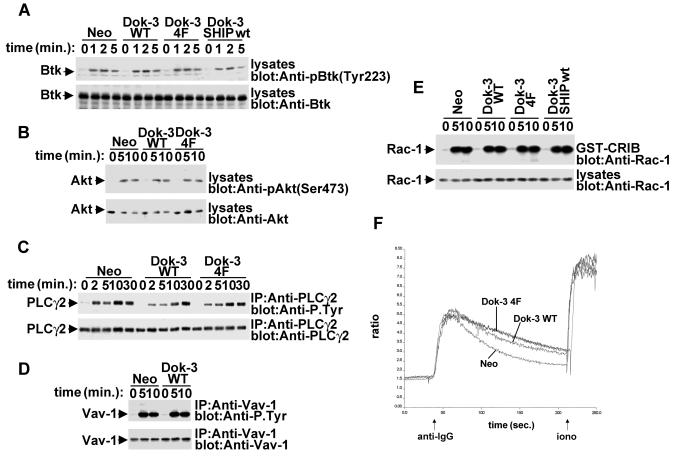
FIG. 6.
Lack of influence of Dok-3 on multiple signaling pathways in A20 B cells. A20 derivatives were stimulated for the indicated periods of time with F(ab′)2 fragments of SAM IgG. (A) BCR-induced Btk activation. Lysates were probed by immunoblotting with phosphospecific anti-Btk antibodies (anti-pY223) (upper block). The abundance of Btk was verified by reprobing of the immunoblot membrane with anti-Btk (lower block). (B) BCR-triggered Akt/PKB activation. Lysates were probed by immunoblotting with phosphospecific anti-Akt antibodies (anti-pS473) (upper block). The abundance of Akt was verified by reprobing of the immunoblot membrane with anti-Akt (lower block). (C) BCR-induced PLC-γ2 tyrosine phosphorylation. PLC-γ2 was immunoprecipitated from cell lysates and probed by immunoblotting with anti-phosphotyrosine (upper block). The abundance of PLC-γ2 was verified by reprobing of the membrane with anti-PLC-γ2. (D) BCR-elicited Vav-1 tyrosine phosphorylation. Vav-1 was immunoprecipitated from cell lysates and probed by immunoblotting with anti-phosphotyrosine (upper block). The abundance of Vav-1 was verified by reprobing of the membrane with anti-Vav-1. (E) BCR-induced Rac-1 activation. Activated Rac-1 was recovered from cell lysates by using a GST fusion protein encompassing the Rac-1-binding region of PAK (GST-CRIB). Associated Rac-1 was detected by immunoblotting with anti-Rac-1. The abundance of Rac-1 in the A20 derivatives was verified by immunoblotting of total cell lysates with anti-Rac-1. (F) BCR-evoked calcium fluxes. Cells were stimulated and calcium fluxes were monitored, as outlined in Materials and Methods.