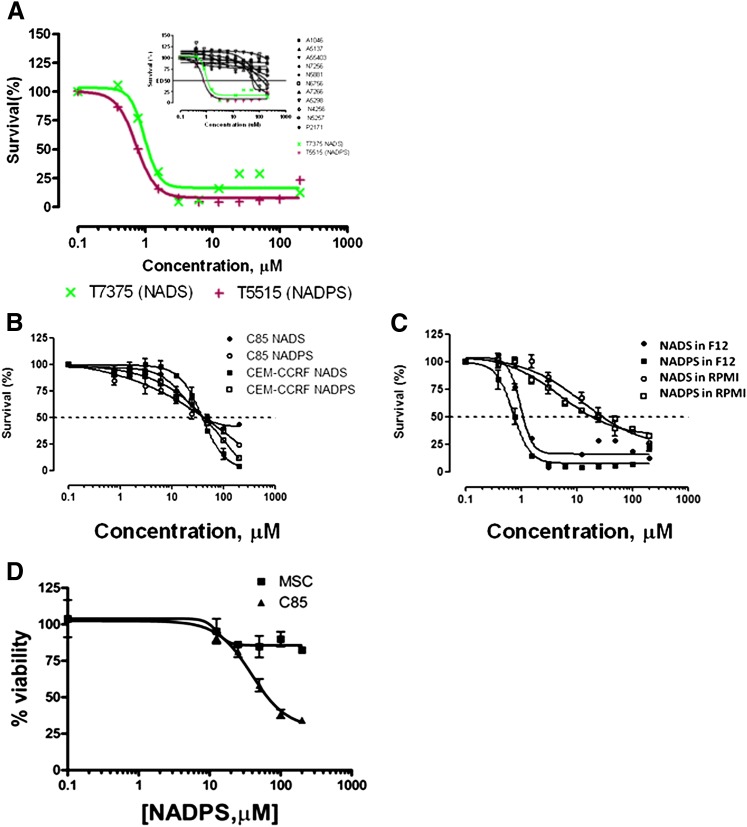
Fig. 1.
Screening of NAD/NADP analogs for cytotoxicity. (A) Two thousand DG44-(DGFR-EGFP) cells per well were plated and grown in Ham’s F12 medium supplemented with 10% dialyzed FBS containing no glycine, hypoxanthine, or thymidine. Concentrations of drugs tested ranged from 200 μM concentration. After incubation with NAD/NADP analogs for 96 hours, the viability of the transfectants was assessed using the MTS proliferation assay (see Materials and Methods for detail). (B) NADS and NADPS were tested in C85 and CCRF-CEM cells in RPMI 1640 medium for 96 hours. Although cell toxicity in C85 cells was determined using MTS assay, Trypan blue exclusion assay was used to determine cell viability in the presence of NADS and NADPS in CCRF-CEM cells. (C) Cytotoxicity of NADS and NADPS was tested for DG44-(DHFR-EGFP) cells in RPMI-1640 and Ham’s F12 using the MTS assay. (D) Seventy-five thousand C85 cells and mesenchymal stem cells (MSCs) were plated into 24-well tissue culture plates in triplicate in RPMI-1640 (C85) or Dulbecco's modified Eagle medium (DMEM) (mesenchymal stem cells) supplemented with 10% FBS. The following day, the medium was removed and replaced with fresh medium containing NADPS. After 96 hours of incubation, the cell viability was determined. The percentage cell survival was determined by Softmax Pro software, and GraphPad Prism 4 software was used to determine ED50 values using a sigmoidal dose-response curve fit.