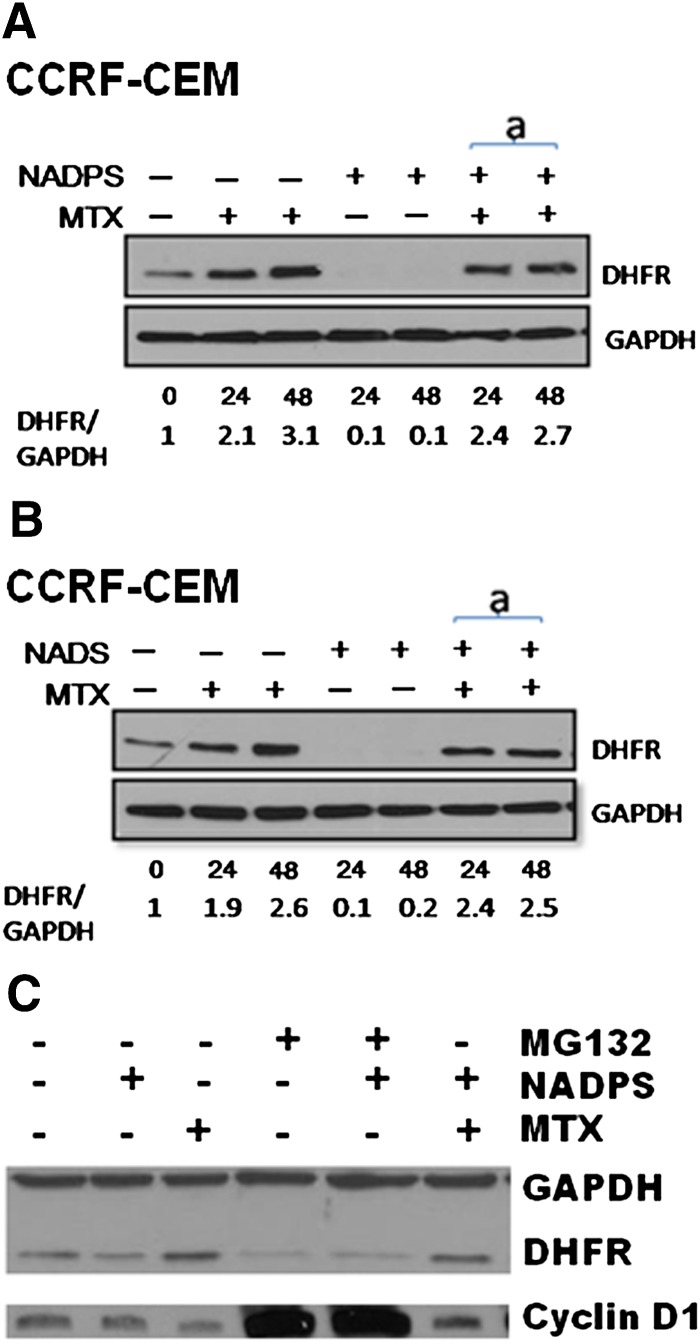
Fig. 5.
A decreased DHFR level by NAD/NADP analogs is attributable to neither inhibition of MTX-mediated translational regulation of DHFR protein nor inhibition of ubiquitin proteasome pathway. DHFR protein levels were determined by Western blotting in CCRF-CEM cells. (A) CCRF-CEM cells were treated with 10 nM MTX alone, 10 μM NADPS alone, and simultaneously with 10 nM MTX and 10 μM NAPDS for 24 and 48 hours. (B) CCRF-CEM cells were treated with 10 nM MTX alone, 10 μM NADS alone, and simultaneously with 10 nM MTX and 10 μM NAPS for 24 and 48 hours. DHFR protein was detected by polyclonal anti-DHFR antibodies, and GAPDH was used as a loading control (A and B). Quantification of DHFR level was shown at the bottom of gels and measured by Image J program (provided by National Institutes of Health). (C) MCF-7 cells treated with 0.5 μM MG132, a proteasome inhibitor, in the presence and absence of 10 μM NADPS for 48 hours. GAPDH was used as the loading control, and Cyclin D1 was used as positive control for the activity of MG132. MCF-7 cells were also treated with MTX in the presence and absence of NADPS and MG132.