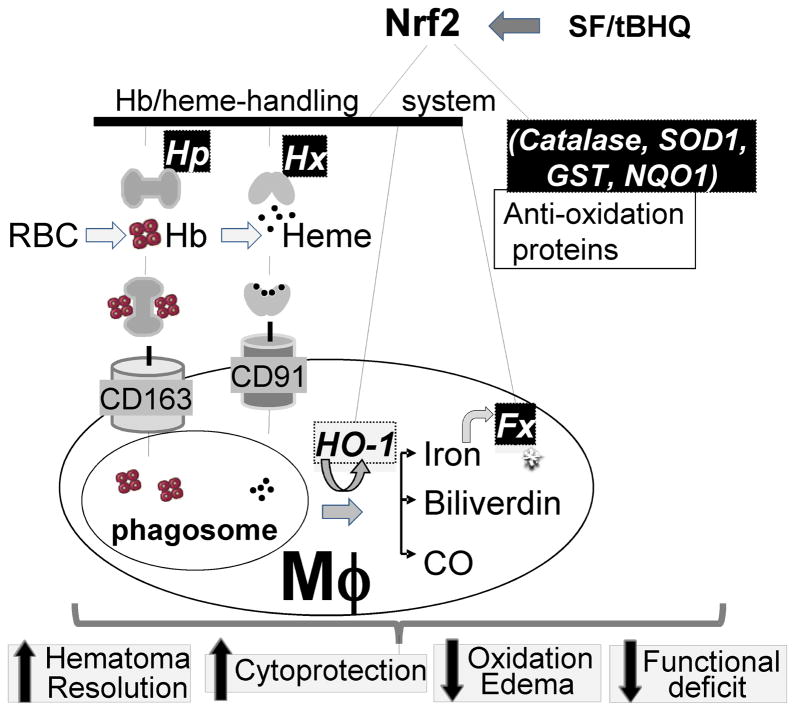
Model 1. The endogenous detoxification system for hemolytic products (Hb, heme and iron).
It is proposed that the cell-free Hb and heme are initially neutralized by binding to Hp and Hx, respectively, forming stable Hb-Hp and heme-Hx complexes. The complexes can be endocytosed by Mφ through the Hb scavenger receptor CD163 [68] and the heme scavenger receptor CD91 [14], respectively. Heme in Mφ is catabolized by HO-1 to biliverdin, carbon monoxide (CO), and free iron [52]. The latter one is sequestrated within Mφ by intracellular iron binding protein, ferritin (Fx). Activation of Nrf2 with SF or tBHQ may upregulate production of: (A) enzymes constituting the antioxidant/cytoprotective defense system (e.g. SOD1, catalase, γGCS, GR, TG, Prxs, GST and NQO1) [69], and (B) detoxification enzymes (e.g. Hp, Hx, HO-1 and Fx) for preventing the toxic effect of hemolytic products. Thus, approaches aiming at stimulating Nrf2 prior to hematoma hemolysis may ameliorate oxidative stress directly by controlling production of anti-oxidant enzymes and indirectly by upregulating components capable of blocking toxic effect of iron-containing hemolytic products.