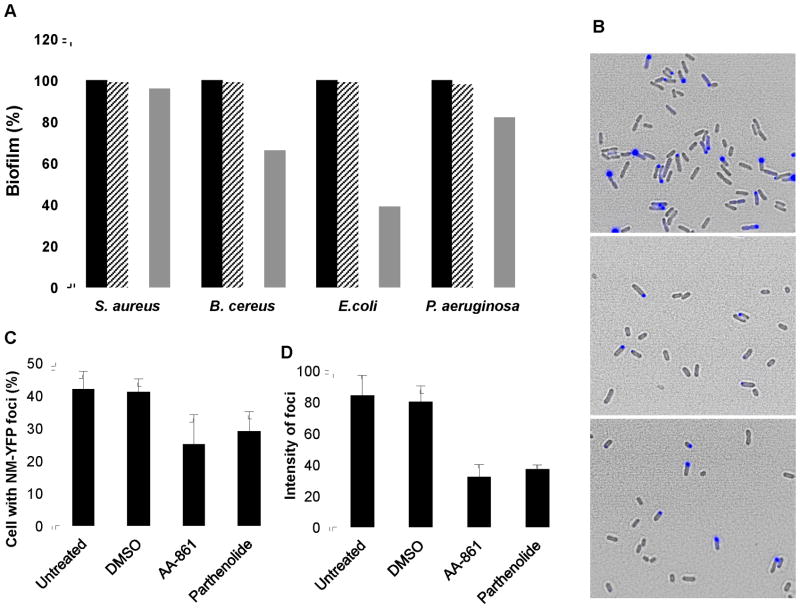
Figure 7. The molecules affect other amyloid proteins.
(A) Anti-biofilm activity against other bacterial species that form surface-adhered biofilms. The crystal violet method was used to quantify biofilm formation. The absorbance at 590 nm was measured and values were represented relative to untreated controls which were normalized to 100%. The molecules were used at a final concentration of 50 μM. Untreated cells (■), treated with DMSO (
 ), AA-861 (□) or parthenolide (
), AA-861 (□) or parthenolide (
 ). (B–D) The molecules reduce the aggregation of New1 yeast prion expressed in E. coli cells. (B) Fluorescence and phase contrast microscopy images were taken of E. coli cells expressing the yeast prion domain New1 fused to CFP and induced with 5 μM IPTG (upper panel). A decrease in the number of cells accumulating New1-CFP foci was observed after treatment with 120 μM of (middle panel) AA-861 and (lower panel) parthenolide. (C) The number of cells accumulating New1-CFP foci was quantified and expressed as percentage of cells with foci, and (D) the average intensity of these foci expressed as arbitrary units.
). (B–D) The molecules reduce the aggregation of New1 yeast prion expressed in E. coli cells. (B) Fluorescence and phase contrast microscopy images were taken of E. coli cells expressing the yeast prion domain New1 fused to CFP and induced with 5 μM IPTG (upper panel). A decrease in the number of cells accumulating New1-CFP foci was observed after treatment with 120 μM of (middle panel) AA-861 and (lower panel) parthenolide. (C) The number of cells accumulating New1-CFP foci was quantified and expressed as percentage of cells with foci, and (D) the average intensity of these foci expressed as arbitrary units.