Abstract
Human bocavirus (HBoV) was recently discovered in children with respiratory distress and/or diarrhea. To our knowledge, no previous study has reported the existence of bocavirus in Saudi Arabia. Swabs samples from 80 children with respiratory tract infections were examined for the presence of HBoV. Real-time polymerase chain reaction was used as a sensitive method to detect the HBoV. Direct gene sequencing was used to determine the genotype of the detected virus isolates. HBoV was detected in 22.5% of the examined patients. The NP1 partial gene sequence from all patients showed that the circulated strains were related to HBoV-1 genotype. Most of HBoV infected patients showed evidence of mixed coinfection with other viral pathogens. The current study clearly demonstrated that genetically conserved HBoV1 circulates in Saudi Arabia. Interestingly, most of the HBoV1 infected cases were associated with high rates of co-infections with other viruses.
Introduction
Bocavirus is a single-stranded DNA virus belonging to the family Parvoviridae, subfamily Parvovirinae, genus Bocavirus. Bocaviruses are unique among parvoviruses because they contain a third ORF between the non-structural and structural coding regions [1]–[2]. The genus bocavirus includes viruses that infect bovine, canine, feline, porcine and some simian species as well as sea lions [3]–[8]. Human bocavirus (HBoV) was first found in children with acute respiratory tract infections in 2005 [1]. It was then detected in children with respiratory tract infections in addition to gastroenteritis worldwide [9]–[12]. The virus exists in four different serotypes HBoV1-4 [1]–[2], [13]–[14]. Although HBoV 1 and 2 were reported in respiratory samples, all the 4 genotypes of HBoV have been identified in children with acute gastroenteritis.
HBoV has been reported in various countries, indicating its worldwide endemic nature. The virus has been identified in Europe [15]–[17], America [18]–[19], Asia [9], [20], Australia [21]–[22], Africa [23], and the Middle East [24]. The prevalence of HBoV ranges between 1.5 to 19.3% [18], [25]. Primary infection with HBoV seems to occur early in life and children between the ages of 6–24 months seem to be mostly affected [9]–[10], but older children can also be infected. Newborn children may become protected by maternally derived antibodies [9]. HBoV infections are rarely found in adults [26]–[27]. Lindner et al. detected anti-HBoV antibodies in 94% of healthy blood donors >19 years of age [28].
HBoV detection has been mostly performed on nasopharyngeal aspirates and swabs and relies mostly on classical [10], [18], [19], [21], [22], [26] and real-time PCR [10], [23], [29]. Real-time PCR possesses many advantages over conventional PCR, as it offers greater sensitivity, specificity, and reduced expenditure of time.
The current study aims to screen the epidemiological status and molecular phylogeny of HBoV isolates prevailing in pediatric patients with respiratory infection in Saudi Arabia.
Results and Discussion
The current study investigated the prevalence of HBoV in patients suffering from respiratory tract infections in Saudi Arabia. The presence of the major viral causes of the respiratory distress in HBoV positive cases was also screened. HBoV was detected in 18/80 of the examined patients (22.5%) with ages ranging from 2 months to 10 years, (Table 1–2). Clinical findings for HBoV-positive patients were indistinguishable from those for patients with other respiratory viruses. Previously, HBoV has been detected in samples from patients aged between 5 months and 2 years [1], [29]. Ma et al, speculated that the antibody against HBoV derived from the mother might protect children under 5 months of age from HBoV infection [9], however, we detected HBoV in two cases below 5 months: in a 2-month-old and 4-month-old child (Table 1–2) that may indicate the possibility of HBoV infection in very young children.
Table 1. Demographic and clinical data of children suffered from respiratory distress in the current study.
| Variable | Value [N = 80] | HBoV infected [N = 18] |
| Age | 2 mo- 10 yr | |
| 0–4 Mo | 11 | 2 |
| 5–8 Mo | 6 | 1 |
| 9–12 Mo | 1 | 1 |
| 1–2 yr | 27 | 8 |
| >2–3 yr | 8 | 4 |
| >3–4 yr | 4 | 0 |
| >4–5 yr | 6 | 1 |
| >5–7 yr | 4 | 0 |
| >7–10 yr | 4 | 1 |
| Sex | ||
| Male | 39 | 8 |
| Female | 41 | 10 |
| Clinical symptoms | ||
| Fever | 80 | 18 |
| Nasal discharge | 80 | 18 |
| Watery | 70 | 16 |
| Purulent | 10 | 2 |
| Asthma | 16 | 2 |
| LRTIa | 41 | 15 |
| URTIb | 39 | 3 |
| Diarrhea | 20 | 3 |
| Nervous manifestations | 2 | 0 |
| Rash | 6 | 0 |
Lower respiratory tract infection.
Upper respiratory tract infection.
Table 2. Common respiratory viruses among HBoV infected children.
| Sample No | Age | Sex | RSV | PIV-1 | PIV-3 | IAV | Adenovirus |
| 1 | 2 Mo | Female | + | − | − | − | − |
| 2 | 10 Mo | Male | − | − | − | − | − |
| 3 | 8 Mo | Male | − | − | − | + | − |
| 4 | 18 Mo | Male | + | − | − | + | − |
| 5 | 19 Mo | Male | + | − | − | − | − |
| 6 | 36 Mo | Female | + | − | + | + | − |
| 7 | 12 Mo | Female | + | − | − | + | + |
| 8 | 36 Mo | Male | + | − | − | − | − |
| 9 | 17 Mo | Female | + | − | − | + | + |
| 10 | 13 Mo | Female | + | − | − | + | + |
| 11 | 5 yr | Female | + | − | − | + | + |
| 12 | 4 Mo | Female | + | − | − | + | + |
| 13 | 20 Mo | Male | + | − | − | + | − |
| 14 | 20 Mo | Male | − | − | − | + | − |
| 15 | 24 Mo | Female | − | − | − | + | + |
| 16 | 10 yr | Female | − | − | − | + | − |
| 17 | 30 Mo | Female | + | − | − | − | − |
| 18 | 32 Mo | Male | + | − | − | − | − |
The rate of HBoV in respiratory tract infections has been reported to be 1.5 to 19.3% [18], [25]. Real-time PCR was used in the current study to screen HBoV due to its high diagnostic sensitivity that could be responsible for the higher rate of HBoV infection in Saudi Arabia than the widely accepted upper limit of infection rates worldwide. Meanwhile, a recent study showed 21.5% prevalence among children [30].
The evidence of HBoV as the main initiator of the disease in the infected cases is still uncertain because of its high co-infection rate with other pathogens, and it remains unclear whether HBoV is the sole etiologic agent or just a concomitant virus bystander. In previous studies, none of the nasal swabs obtained from healthy children yielded a positive HBoV test. This suggests that HBoV is not a frequent commensal virus inhabiting the respiratory tract [16], [19]. HBoV infections are frequently present in concomitant with other viruses and often occur in more than 50% of the tested samples [31]. In the current study, only one case was found to be infected only by HBoV as a single virus entity while most of isolates (17/18) showed coinfection with other viral pathogens. The most frequently detected co-pathogens were RSV (13/18; 72.2%), IAV (12/18 cases, 66.66%), respiratory adenovirus (6/18 cases, 33.33%) while only 1/18 (5.5%) case was coinfected with PIV-3 and none was coninfected with PIV-1 (Table 2). It is assumed that the rate and frequency of coinfections may be higher if more viruses were screened. Consistent with other studies [1], [16], the prevalence rate of bocavirus was higher in children under 2 years of age (Table 1).
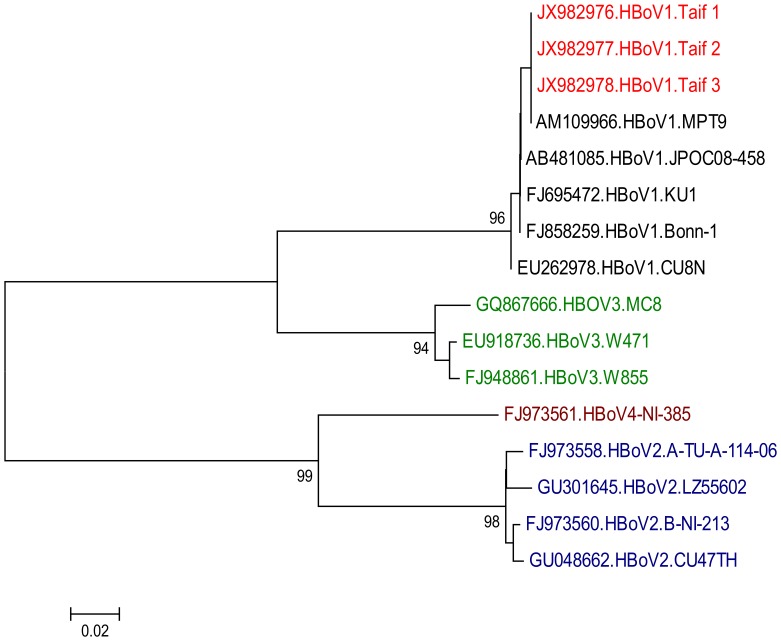
Partial NP-1 gene sequence of the eighteen detected HBoV strains were obtained in our study. Multisequence analysis showed complete identity (100%) between each other, and phylogenetic analysis demonstrated that they belonged to HBoV1 (data not shown). Blast analysis revealed complete homology to the published sequence of HBoV1. Furthermore, the phylogenetic analysis results of three selected sequences showed that the Saudi HBoV1 strains obtained from respiratory samples belonged to group I human bocaviruses (Fig. 1).
Figure 1. Phylogenetic analysis of Saudi HBoV1 isolates.
The phylogenetic tree with 1,000 bootstrap replicates was generated using the Clustal W program in the MEGA 4.0 software package and based on partial NP-1 sequences of the HBoV strains. Samples obtained in the current study from children with acute respiratory distress in Saudi Arabia are in red. HBoV1 strains are presented in black, HBoV 2 strains are presented in blue, HBoV3 strains are presented in green while HBoV4 isolate is presented in brown.
To the best of our knowledge, this is the first report of HBoV1 in Saudi Arabia. Continuous surveillance and genome sequence analysis are needed to obtain more information on the genotypic variation and molecular evolution of HBoV in the country.
Materials and Methods
Ethics Statement
The study protocol was approved by the medical ethics review board of the College of Medicine, Taif University and by the pediatric hospital ethics committee in accordance with the guidelines for the protection of human subjects. Informed written consents from the next of kin of the participants involved in the study were taken.
Sample Collection and Processing
Nasopharyngeal swabs from 80 children suffered from moderate to severe lower respiratory tract infections were collected from January to May 2012 from the Governmental Pediatric Hospital- Al-Taif, Saudi Arabia. The children’s age ranged from two months to ten years of age. Clinical manifestations and case histories were recorded. Individual swabs were kept in 1 ml sterile saline containing gentamycin sulphate. Swabs were routinely processed and kept at −80°C until further analysis.
Nucleic Acid Extraction
Viral nucleic acid was extracted from 200 µl of individual samples using DNA/RNA extraction Kit (Koma Bioteck Inc., Seoul, Korea), according to the manufacturer’s instructions.
Real Time Polymerase Chain Reaction (RT PCR)
The real-time PCR assay was performed using commercial, TaqMan hydrolysis probe based, real time PCR bocavirus detection Kit (Liferiver, Shanghai, China) in Eppendorf Mastercycler® ep realplex2. The detection of the amplified amplicon was performed in fluorimeter channel FAM with the fluorescent quencher BHQ1. Amplification reactions were performed in a volume of 25 µl containing 2.5 µl of DNA template, 21.5 µl reaction mix, 0.4 µl enzyme mix, 1 µl internal control according to the manufacturer’s instructions. The thermal cycling conditions were as follows: 2 min at 37°C, an initial denaturation of 2 min at 94°C and 40-cycles of 15 sec at 93°C and annealing/elongation step of 1 min at 60°C. HBoV positive samples were screened for the presence of respiratory syncytial virus (RSV), influenza A virus (IAV), parinfluenza virus 1(PIV-1) and parainfluenza virus 3(PIV-3), as well as respiratory enteric virus (AdenV) using real-time PCR Kits (Shanghai ZJ Bio-tech Co., Ltd).
HBoV Genotyping
PCR amplification of a 354–base pair fragment of the NP1 was performed as described previously [1]. The reaction mix contained 20 pmol of each primer and DNA master mix (Koma Bioteck, Inc., Seoul, Korea). The thermal cycling conditions were as follows: an initial denaturation of 5 min at 94°C, 35 cycles of 1 min at 94°C, 1 min at 54°C and 2 min at 72°C, final extension of 10 min at 72°C.Positive PCR products were purified using a QIAquick PCR purification kit (Qiagen) and were sequenced commercially (Macrogen Inc., Seoul, Korea).
Sequence Analysis
The nucleotide sequences of the NS1 gene were compared with those of HBoV strains available at the GenBank site. Phylogenetic analyses were conducted with MEGA, version 4.1. The 3/18 partial sequences of the NS1 gene were submitted to GenBank (accession numbers JX982976–JX982978).
Funding Statement
This study was supported by a research grant (Project No. 1652-433-1) through the Research Support of Taif University, Saudi Arabia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, et al. (2005) Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA 102: 12891–12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kapoor A, Simmonds P, Slikas E, Li L, Bodhidatta Let al (2010) Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J Infect Dis 201: 1633–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claude M, Fauquet MAM, Maniloff J, Desselberger U, Ball LA (2004) Virus Taxonomy: The Eighth Report of the International Committee on Taxonomy of Viruses. Academic Press. [Google Scholar]
- 4. Cheng WX, Li JS, Huang CP, Yao DP, Liu N, et al. (2010) Identification and nearly full-length genome characterization of novel porcine bocaviruses. PLoS One 5: e13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kapoor A, Mehta N, Esper F, Poljsak-Prijatelj M, Quan PL, et al. (2010) Identification and characterization of a new bocavirus species in gorillas. PLoS One 5: e11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharp CP, LeBreton M, Kantola K, Nana A, Diffo Jle D, et al. (2010) Widespread infection with homologues of human parvoviruses B19, PARV4, and human bocavirus of chimpanzees and gorillas in the wild. J Virol 84: 10289–10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li L, Shan T, Wang C, Côté C, Kolman J, et al. (2011) The fecal viral flora of California sea lions. J Virol 85(19): 9909–9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lau SK, Woo PC, Yeung HC, Teng JL, Wu Y, et al. (2012) Identification and characterization of bocaviruses in cats and dogs reveals a novel feline bocavirus and a novel genetic group of canine bocavirus. J Gen Virol 93: 1573–1582. [DOI] [PubMed] [Google Scholar]
- 9. Ma X, Endo R, Ishiguro N, Ebihara T, Ishiko H, et al. (2006) Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol 44: 1132–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manning A, Russell V, Eastick K, Leadbetter GH, Hallam N, et al. (2006) Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis 194: 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vicente D, Cilla G, Montes M, Perez-Yarza EG, Perez-Trallero E (2007) Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis 13: 636–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng WX, Jin Y, Duan ZJ, Xu ZQ, Qi HM, et al. (2008) Human bocavirus in children hospitalized for acute gastroenteritis: a case-control study. Clin Infect Dis 47(2): 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arthur JL, Higgins GD, Davidson GP, Givney RC, Ratcliff RM (2009) A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog 2009 5: e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapoor A, Slikas E, Simmonds P, Chieochansin T, Naeem A, et al. (2009) A newly identified bocavirus species in human stool. J Infect Dis 199: 196–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foulongne V, Olejnik Y, Perez V, Elaerts S, Rodiere M, et al. (2006) Human bocavirus in French children. Emerg Infect Dis 12: 1251–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maggi F, Andreoli E, Pifferi M, Meschi S, Rocchi J, et al. (2007) Human bocavirus in Italian patients with respiratory diseases. J Clin Virol 38: 321–325. [DOI] [PubMed] [Google Scholar]
- 17. Regamey N, Frey U, Deffernez C, Latzin P, Kaiser L (2007) Isolation of human bocavirus from Swiss infants with respiratory infections. Pediatr Infect Dis J 26: 177–179. [DOI] [PubMed] [Google Scholar]
- 18. Bastien N, Brandt K, Dust K, Ward D, Li Y (2006) Human Bocavirus infection, Canada. Emerg Infect Dis 12: 848–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kesebir D, Vazquez M, Weibel C, Shapiro ED, Ferguson D, et al. (2006) Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis 194: 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin F, Zeng A, Yang N, Lin H, Yang E, et al. (2007) Quantification of human bocavirus in lower respiratory tract infections in China. Infect Agent Cancer 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM (2006) Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol 78: 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sloots TP, McErlean P, Speicher DJ, Arden KE, Nissen MD, et al. (2006) Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol 35: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smuts H, Hardie D (2006) Human bocavirus in hospitalized children, South Africa. Emerg Infect Dis 12: 1457–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaplan NM, Dove W, Abu-Zeid AF, Shamoon HE, Abd-Eldayem SA, et al. (2006) Human bocavirus infection among children, Jordan. Emerg Infect Dis 12: 1418–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonzel L, Tenenbaum T, Schroten H, Schildgen O, Schweitzer-Krantz S, et al. (2008) Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J 27: 589–594. [DOI] [PubMed] [Google Scholar]
- 26. Kupfer B, Vehreschild J, Cornely O, Kaiser R, Plum G, et al. (2006) Severe pneumonia and human bocavirus in adult. Emerg Infect Dis 12: 1614–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu L, He X, Zhang DM, Feng FS, Wang Z, et al. (2012) Surveillance and genome analysis of human bocavirus in patients with respiratory infection in guangzhou, china. PLoS One 7(9): e44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lindner J, Karalar L, Zehentmeier S, Plentz A, Pfister H, et al. (2008) Humoral immune response against human bocavirus VP2 virus-like particles. Viral Immunol 21: 443–449. [DOI] [PubMed] [Google Scholar]
- 29. Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, et al. (2007) Human bocavirus and acute wheezing in children. Clin Infect Dis 44: 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ghietto LM, Cámara A, Zhou Y, Pedranti M, Ferreyra S, et al. (2012) High prevalence of human bocavirus 1 in infants with lower acute respiratory tract disease in Argentina, 2007–2009. Braz J Infect Dis 16(1): 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christensen A, Nordbø SA, Krokstad S, Rognlien AG, Døllner H (2008) Human bocavirus commonly involved in multiple viral airway infections. J Clin Virol 41: 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]