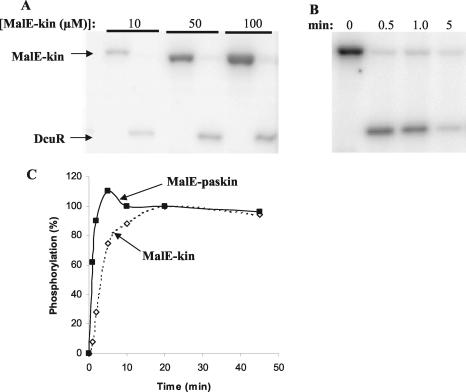
FIG. 7.
Autophosphorylation of the DcuS-kinase domain and phosphotransfer to DcuR. (A and B) Phosphorimager-generated autoradiographs of phosphorylated DcuS (MalE-Kin) and DcuR separated by SDS-PAGE (reactions performed at 25°C). DcuS was phosphorylated with [γ-32P]ATP prior to the addition of DcuR. (A) DcuR (5 μM) was added to phosphorylated DcuS at time zero, resulting in the complete dephosphorylation of DcuS within 1 min and concomitant phosphotransfer to DcuR. The level of DcuR phosphorylation increased with increasing concentrations of phosphorylated DcuS. (B) DcuR (5 μM) was added to 20 μM phosphorylated DcuS at time zero. After the initial rapid phosphotransfer from DcuS to DcuR, the phosphorylated form of DcuR dephosphorylated, resulting in the almost complete dephosphorylation of DcuR after a 5-min incubation. (C) Graph comparing the relative rates of autophosphorylation for MalE-Kin and MalE-PAScKin at 25°C. Reactions for the two DcuS proteins were performed in the same tube, and the degree of phosphorylation was determined (as a percentage of the level achieved at 20 min) after the separation of the two proteins by SDS-PAGE. Note that the two proteins incorporated closely similar amounts of 32P after 20 and 45 min of incubation.