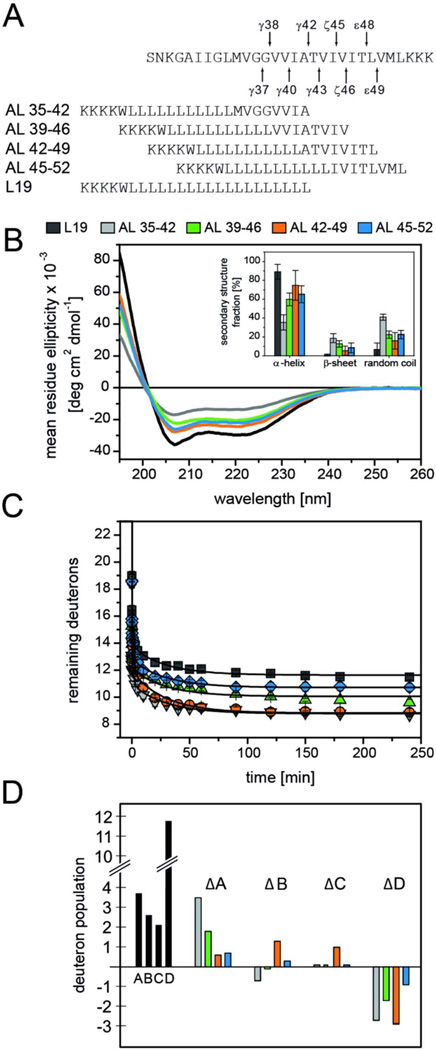
Figure 4. Secondary structure and backbone dynamics of hybrid peptides.
A) Peptide sequences. B) CD spectra and calculated secondary structure contents (inset) of AL-peptides in DLPC/DLPS/DLPE (3/1/1) membranes at P/L ~0.03 in 50 mM ND4Ac, pD 7.5 at 70°C. C) DHX kinetics of AL-peptides recorded in liposomal DLPC/DLPS/DLPE (3/1/1) membranes at P/L ~0.03 in 50 mM NH4Ac, pH 7.5 at 70°C. Data points at t=0 min correspond to the numbers of amide deuterons seen after exchange under quench conditions. Lines connecting the data points were obtained by fitting the kinetics with a triple exponential function that was characterized by a reduced chi-square value <0.1 for all peptides. n = 3, means±SD. D) A representation of the numbers of deuterons within the kinetically distinct classes A, B, C, and class D, which represents deuterons that do not exchange within 4 h. To make them comparable, the numbers were calculated after averaging the respective DHX rate constants over all peptides (Table S1). Black bars represent the numbers found for the L19 reference. To facilitate the comparison between AL peptides, we plot the differences between the numbers seen with AL-peptides and L19.