Table 1. Crosslinking agents used in the clinic.
| Drug | Clinical application | Dose-limiting toxicity | Example solution structure |
|---|---|---|---|
| Platinums | |||
| Cisplatin | Testicular, ovarian and non-small-cell lung cancer |
Central nervous system, renal and gastrointestinal toxicity153 |
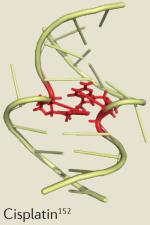
|
| Carboplatin | Ovarian cancer | Neutropenia154 | |
| Oxaliplatin | Colorectal cancer | Neuropathy135,155 | |
| Satraplatin | Prostate and breast cancer | Thrombocytopenia and neutropenia156,157 |
|
| Picoplatin | Phase II trials for relapsed lung cancer and Phase I and II trials for the treatment of solid tumours, and prostate, colorectal and small-cell lung cancer |
Thrombocytopenia and neutropenia158 |
|
| Nitrogen mustards | |||
| Cyclophosphamide | Lymphoma | Neutropenia160 |
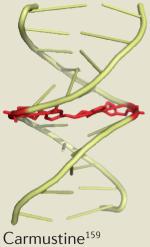
|
| Melphalan | Multiple myeloma, melanoma and ovarian cancer |
Leukopenia and thrombocytopenia161 |
|
| Chlorambucil | Chronic lymphocytic leukaemia | Pancytopenia and neurotoxicity162 |
|
| Ifosfamide | Non-small-cell lung cancer | Leukopenia, thrombocytopenia and renal toxicity163 |
|
| Others | |||
| Mitomycin C | Oesophageal and bladder cancer | Leukopenia and thrombocytopenia165,166 |
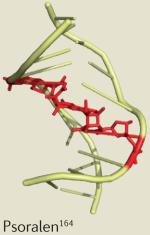
|
| Psoralen plus ultraviolet A radiation |
Cutaneous T cell lymphoma | Dermatitis167,168 | |
| Pyrrolobenzodiazepines | Phase II trial for solid tumours | Fatigue and thrombocytopenia169 |
|
