Summary
Background
The vascular endothelium plays an integral role in maintaining vascular homeostasis, including the regulation of blood flow, vascular tone, and platelet aggregation. The aim of this study was to see if there were any differences in endothelial function between Koreans and Caucasians.
Material/Methods
This was accomplished by 2 measures of endothelial function – the response to local heat and the response to vascular occlusion. Ten Caucasian and 10 Korean male and female subjects participated (<35 years old). Endothelial function was assessed by the skin blood flow response to local heat using a thermode for 6 minutes at 3 temperatures (38°C, 40°C and 42°C) and by vascular occlusion for 4 minutes followed by release and measurement of skin blood flow for 2 minutes.
Results
When applying 6 minutes of local heat at 3 different temperatures (38°C, 40°C, and 42°C), the skin blood flows were significantly higher for all temperatures in Caucasians as compared with Koreans, with peak blood flow of 223±48.1, 413.7±132.1, and 517.4±135.8 flux in Caucasians and 126.4±41.3, 251±77.9, and 398±97.2 flux in Koreans, respectively (p=0.001). Results of this study support the idea that the skin blood flow response to occlusion was significantly higher in Caucasians (peak 411.9±88.9 flux) than Koreans (peak 332.4±75.8 flux) (p=0.016).
Conclusions
These findings suggest that Koreans may have lower endothelial function than Caucasians, which may be explained, in part, by genetic variations between the 2 ethnic groups.
Keywords: endothelial function, local heat, blood flow, ethnicity
Background
The vascular endothelium is a single layer of cells covering the internal surface of blood vessels in the body. It plays an important role in vascular growth, vasoregulation and vasoprotection [1]. Over the past 3 decades, endothelial function has emerged as a key topic because endothelial dysfunction is a leading cause of cardiovascular and age-related diseases and appears to be an independent predictor of these diseases [2]. Therefore, the evaluation of endothelial function is very important and meaningful in the clinical setting. The most common clinical method for assessing endothelial function is post-ischemic reactive hyperemia [3–6]. This is a noninvasive method for assessing and monitoring endothelial function in various populations and disease states, suitable for use in clinical practice [3,5]. Another method of assessing endothelial function involves assessing the skin blood flow response to a heat source [7–9]. The 2 methods differ in that they test different metabolic pathways in endothelial cells. Studies looking at endothelial function, however, have been largely conducted in Caucasians and not much is known about endothelial function in Koreans or other Asian populations.
The vascular endothelium plays an integral role in maintaining vascular homeostasis, including the regulation of blood flow, vascular tone and platelet aggregation [10]. A balance between endothelium-derived relaxing and contracting factors maintains vascular homeostasis [11]. Endothelium-derived relaxing factors are mainly nitric oxide (NO) and prostacyclin (PGI2). Nitric oxide is produced by the enzymatic conversion of L-arginine to L-citrulline by nitric oxide synthase and possesses vasodilating, antithrombotic, and anti-proliferative properties. Prostacyclin (PGI2), with similar biological effects as nitric oxide, is synthesized from arachidonic acid [3,10]. Endothelial dysfunction, which is the disruption of a balance between vasodilation and vasoconstriction, is common with aging and diabetes. While there is little impairment in the ability to vasoconstrict, diminished nitric oxide bioavailability and reduced prostacyclin cause a shift in vascular homeostasis from vasodilation toward vasoconstriction [11]. Degradation of nitric oxide and prostacyclin by reacting with free radicals is a major cause of endothelial dysfunction [12].
The most common test of endothelial function is assessment of post-ischemic reactive hyperemia [1,6]. The reactive hyperemia to anoxia is the sudden rise in blood flow, which can be measured by a Laser Doppler Imager or ultrasound after a 4-minute occlusion of arterial blood flow [13]. The reactive hyperemia is a result, in part, of myogenic and/or metabolic factors, including adenosine, nitric oxide, and prostaglandins. Among them, vasodilator prostaglandins are an essential mediator of reactive hyperemia in the human skin [3,4,6]. In contrast, nitric oxide does not play a major role in causing vasodilation during peak reactive hyperemia in the human skin [3,6]. However, although nitric oxide does not directly mediate reactive hyperemia in the skin, the possibility exists that NO could act in conjunction with 1 or more vasodilators to mediate the reactive hyperemia [3,6]. Several investigators found that inhibition of prostaglandin synthesis significantly reduced the peak hyperemic response but had no effect on the total hyperemic response [3,6]. However, when prostaglandin synthesis inhibition and nitric oxide synthesis inhibition were administered simultaneously, the total hyperemic response was significantly reduced but not eliminated [3,6]. This suggests that prostacyclin and nitric oxide act synergistically to cause vasodilation during reactive hyperemia, but other mediators are involved [3,6]. With clinical relevance, lower blood flow responses to vascular occlusion have also been found in aging, diabetes, smoking, and cardiovascular diseases [13,14].
Assessment of the skin blood flow response to local heat provides a convenient method of evaluating endothelial function [8,9]. Local heat application to the skin results in a large increase in skin blood flow that is biphasic and characterized by a rapid initial peak followed by a more prolonged plateau [14–17]. The initial peak of blood flow response to local heat is primarily mediated by an axon reflex mechanism through the release of substance P and calcitonin gene-related peptide. The secondary prolonged plateau of thermal hyperemic response is mediated primarily by nitric oxide synthase-mediated generation of nitric oxide [8,14,16–18]. However, due to impairment of endothelial function to the nitric oxide pathway and also to the prostacyclin pathway, caused by free radicals associated with aging and diabetes, the blood flow response to local heat is reduced as an individual ages and in diabetics [14,19].
Both of these stressors test endothelial function, but in different ways. Further, heat exposure is a practical issue in our daily lives and has tremendous clinical relevance. However, although the blood flow response to vascular occlusion and local heat has been well documented in Caucasians, little work has been done in Koreans or other Asians. According to a previous study, the blood flow response to occlusion is blunted in Asians compared to Caucasians, possibly due to special genes called thrifty genes, which can affect endothelial function [20]; these genes are seen in Koreans. Therefore, the purpose of this study was to compare differences in endothelial function by assessing the blood flow response to local heat and vascular occlusion in Koreans compared to Caucasians.
Material and Methods
Subjects
Healthy subjects were recruited and assigned to 2 groups based on self-reported ethnicity. Subjects did not have diagnosed cardiovascular disease, hypertension (>140/90 mmHg) or diabetes, were non-smokers, were not taking any medications that would affect the cardiovascular system, and did not have any known peripheral circulatory diseases. The subjects included 10 Caucasians and 10 Koreans. The general characteristics of the subjects are shown in Table 1. There was no significant difference in age, height, weight, body mass index (BMI), skin thickness, subcutaneous fat thickness, and skin moisture between the 2 ethnic groups. All protocols and procedures were approved by the Institutional Review Board of Loma Linda University and all subjects signed a statement of informed consent.
Table 1.
Mean (SD) of general characteristics of the 5 men and 5 women in each ethnic group.
| Caucasians | Korean | p-value | |
|---|---|---|---|
| Age (years) | 27.8 (2.4) | 25.4 (4.2) | 0.14 |
| Height (cm) | 176.1 (11.3) | 168.8 (9.4) | 0.14 |
| Weight (kg) | 73.6 (8.3) | 70.5 (16.7) | 0.60 |
| BMI (kg/m2) | 23.8 (1.8) | 24.5 (3.9) | 0.60 |
| Skin thickness (cm) | 0.05 (0.01) | 0.05 (0.01) | 0.51 |
| Fat thickness (cm) | 0.11 (0.01) | 0.10 (0.01) | 0.15 |
| Skin moisture (mg/cm2) | 40.8 (12.2) | 41.4 (6.3) | 0.89 |
Methods
Measurement of skin blood flow
Skin blood flow was measured with a MOOR Laser Doppler Imager (Moor LTD, Oxford, England). The Imager used a red laser beam to measure blood flow in the skin. The laser was used in a single-spot mode, which was left on the skin and the reflected energy was used to measure the skin blood flow. Blood flow as measured by a Laser Doppler Imager was expressed in a unit called flux. The laser was warmed for 30 minutes prior to taking measurements. The stated reliability from the manufacturer is ±5% from day-to-day. Possible potential sources of this reliability are age and internal temperature of this machine. The laser was calibrated before, in the middle and at the end of the study; there were no calibration changes noted.
Measurement of skin temperature
Skin temperature was measured with a thermistor manufactured by BioPac Systems (BioPac Inc., Goleta, CA). The thermistor output was amplified with an SKT 100 Thermistor amplifier (BioPac systems, Goleta, CA) and the output was then digitized at 1000 samples per second on a BioPac MP150 data collection system at 24 bits of resolution (BioPac Systems, Goleta, CA).
Control of skin temperature
Skin temperature was controlled with a thermode. The thermode consisted of a plastic box with a port on each end so that water could move through the box. The box was approximately 5×2.5×2.5 cm in size. On each end of the box there was a thermocouple such that as water circulated through the box from a controlled temperature water bath (BioPac Systems, Goleta, California). The temperature difference across the box was measured. Water bath temperatures were kept at 38°C, 40°C, or 42°C. A final hole through the box, which was water insulated, allowed the laser to scan through the center of the box onto the skin. This then provided blood flow data while the skin temperature was clamped at a set temperature. Further details on the technique and its reliability and validity have been published elsewhere [21,22].
Measurement of subcutaneous fat and skin thickness
Subcutaneous fat and skin thickness were measured with a Mindray M7 (Mindray, Shanghai, China) using a linear L34256-element probe at a frequency of 10 MHz. A 0.5-cm standoff was used under the probe, and the probe was held vertical (90° to the skin) to avoid false echoes in the measurement of skin and subcutaneous fat thickness.
Procedures
Subjects were interviewed for inclusion and exclusion criteria. Those subjects that were eligible were enrolled into the study and read and signed the informed consent. Next, subjects rested for 15 minutes while skin and fat thickness, height, and weight were taken. Baseline skin blood flow was recorded for 1 minute. After this period of time, the thermode was applied upon the arm above the brachioradialis muscle to warm the skin to 38°C, 40°C, or 42°C on 3 separate days. The thermode was left on for 6 minutes. On another day, occlusion was applied by a blood pressure occlusion cuff inflated to 200 mmHg for 4 minutes followed by 2 minutes of additional blood flow recording. Skin temperature at this site was measured throughout the experimental period. Each experiment took approximately 10 minutes and was performed on 4 separate days.
Statistical analysis
Baseline characteristics of Caucasians and Koreans were compared using an independent t-test (Table 1). Means and standard deviations of skin blood flow were calculated before and after 4 minutes of vascular occlusion and 6 minutes of thermode at different temperatures. A mixed factorial ANOVA was conducted to compare the effect of different temperatures and vascular occlusion on blood flow between Caucasians and Koreans over time. The level of significance was set at p=0.05.
Results
Blood flow response to vascular occlusion
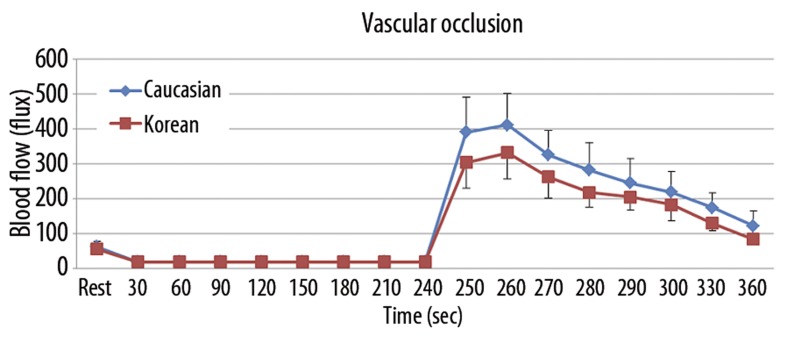
The results of skin blood flow during the 4 minutes of vascular occlusion and then the first 2 minutes following occlusion are shown in Figure 1. There was a significant difference in the total blood flow response between minutes 4 and 6 between Caucasians and Koreans (p=0.016). The mean blood flow rapidly increased to a peak of 411.9±88.9 flux in Caucasians compared to 332.4±75.8 flux in Koreans at 20 seconds after releasing 4 minutes of vascular occlusion. The blood flow then decreased to a final value of 122.3±42.4 flux in Caucasians compared to 84.8±15.5 flux in Koreans at the end of the 2-minute period.
Figure 1.
Mean ±SD of blood flow (flux) measured during the 4 minute period of occlusion and the 2 minute period following the release of the occlusion cuff in 10 Caucasians and 10 Koreans.
Blood flow response to local heat
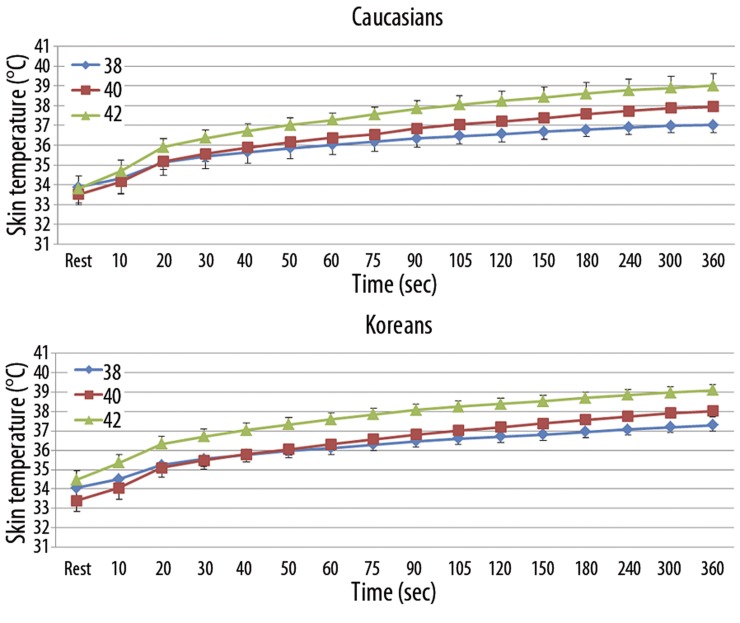
When applying local heat on the forearm, skin temperature and skin blood flow were measured. When heat was applied on the forearm skin, temperature significantly increased continually to a peak skin temperature at the 3 different thermode temperatures (38°C, 40°C, and 42°C) in Caucasians and Koreans (p<0.001) (Figure 2). However, skin temperature at rest and throughout the heat exposure (6 minutes) was not significantly different between Caucasians and Koreans at all 3 different thermode temperatures.
Figure 2.
Mean ±SD of skin temperature (°C) recorded throughout the exposure to a 38, 40, 42°C thermode applied to the skin for period of 360 seconds in 10 Caucasians and 10 Koreans.
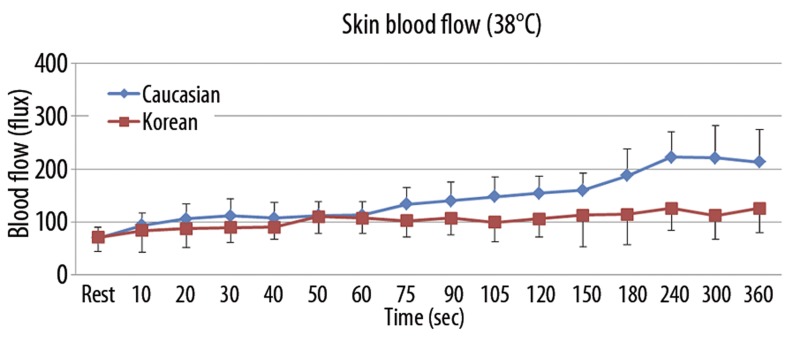
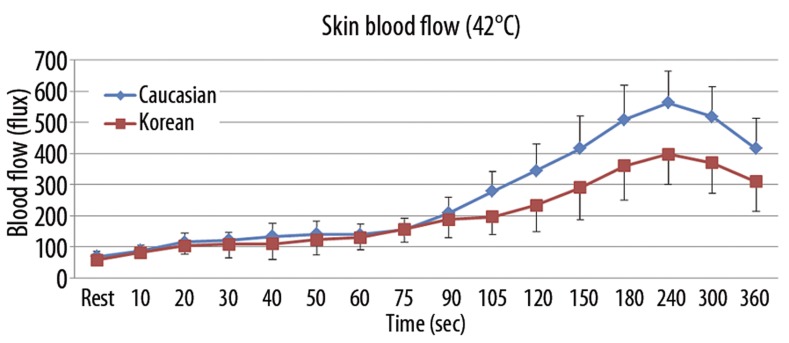
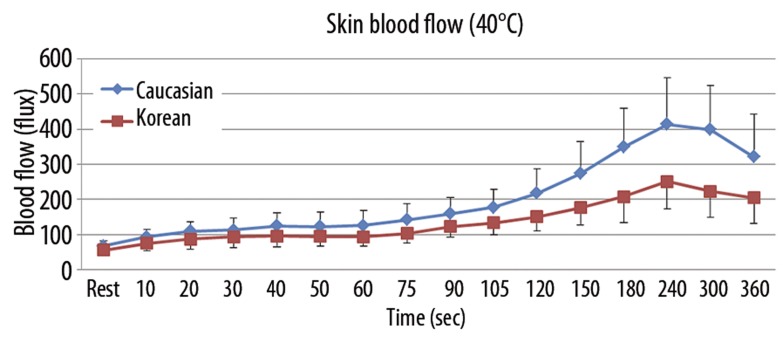
The results of skin blood flow during 6 minutes of heat exposure at 3 different thermode temperatures (38°C, 40°C, and 42°C) in both Caucasians and Koreans are shown in Figures 3–5. When applying 6 minutes of local heat on the forearm at 3 different thermode temperatures (38°C, 40°C, and 42°C), skin blood flow continuously increased in both groups to the peak point at 240 seconds after the heat was applied and then decreased afterward (p=0.001). The total skin blood flow over the 6-minute period at all 3 different thermode temperatures was significantly higher in Caucasians than Koreans (p=0.001). For the 38°C thermode, the skin blood flow was significantly higher in Caucasians than in Koreans after 60 seconds of heat exposure (p=0.004). The peak blood flow after heat exposure was 223.0±48.1 flux in Caucasians and 126.4±41.3 flux in Koreans at 240 seconds (Figure 3). For exposure to the 40°C thermode, the skin blood flow was significantly higher in Caucasians than in Koreans after 60 seconds of heat exposure (p=0.003). The peak blood flow after heat exposure was 413.7±132.1 flux in Caucasians and 251.0±77.9 flux in Koreans at 240 seconds (Figure 4). For exposure to the 42°C thermode, the skin blood flow was significantly higher in Caucasians than in Koreans after 90 seconds of heat exposure (p=0.015). The peak blood flow after heat exposure was 517.4±135.8 flux in Caucasians and 398.0±97.2 flux in Koreans at 240 seconds (Figure 5).
Figure 3.
Mean ±SD of blood flow (flux) measured during the exposure to heat at 38°C in 10 Caucasians and 10 Koreans at rest and over a period of 360 seconds.
Figure 5.
Mean ±SD of blood flow (flux) measured during the exposure to heat at 42°C in 10 Caucasians and 10 Koreans at rest and over a period of 360 seconds.
Figure 4.
Mean ±SD of blood flow (flux) measured during the exposure to heat at 40°C in 10 Caucasians and 10 Koreans at rest and over a period of 360 seconds.
Discussion
Endothelial dysfunction implies an imbalance between endothelium-derived relaxing and contracting factors maintaining vascular homeostasis. Endothelial dysfunction plays an important role in the pathogenesis of most known cardiovascular diseases and diabetes mellitus and is an independent predictor of future cardiovascular diseases [8,23]. The human skin circulation is a useful and an appropriate model for generalized microvascular endothelial function [24]. The skin blood flow response to reactive hyperemia following occlusion and local heating are commonly used to evaluate microvascular endothelial function in normal subjects and patients with cardiovascular diseases, diabetes mellitus, and other stressors that affect the blood flow in the body [6,24].
The present study examined vascular endothelial function in 10 young Caucasian subjects and 10 young Korean subjects. Endothelial function was assessed by the blood flow response to both occlusion and local heating. In the reactive hyperemia after 4 minutes of vascular occlusion, the skin blood flow response to occlusion was significantly higher in Caucasians than Koreans. In the response to 6 minutes of local heating at 3 different thermode temperatures (38°C, 40°C and 42°C), the skin blood flow response to local heating was significantly higher in Caucasians than Koreans. These findings suggest that the Korean population has lower vascular endothelial function than Caucasians. Several previous studies have demonstrated reduced or impaired vascular endothelial function in Asians compared with Caucasians [25,26]. However, this study is the first to compare endothelial function between Koreans and Caucasians by measuring the skin blood flow response to both occlusion and local heating. The ethnic differences in endothelial function, particularly in the bioavailability of endothelium-derived nitric oxide, have been suggested to play an important role. These differences are likely to be clinically important because endothelium-derived nitric oxide plays a major role in vascular homeostasis as a vasodilator and as an inhibitor of platelet activity, monocyte adhesion, and smooth muscle proliferation [11]. Furthermore, a recent study has demonstrated that impaired endothelial function in the microvasculature is predictive of future cardiovascular disease events [27]. According to recent studies, the predicted prevalence of metabolic syndrome is 25% for non-Hispanic whites compared to 45% of Asians (Korean, Asian Indian, Chinese, Filipino, Japanese, Vietnamese) and prevalence of diabetes in Asian Americans was 60% higher than in non-Hispanic whites [28,29]. Such ethnic difference could be either due to a natural lower blood flow in Koreans or the influence of the thrifty genotype on endothelial function [25,30,31]. Due to a modern high fat diet, this genotype may have caused endothelial damage.
The thrifty genotype developed in populations where food supply was limited due to famine. People with thrifty genes could store fat better than others and were therefore more likely to survive prolonged food shortages [20]. This thrifty genotype, which is composed of many single nucleotide polymorphisms (SNPs), is a genetic difference regulating lipid metabolism and fat storage, and is different depending on ethnicity [25,26,30,31]. For example, one of the thrifty SNPs, a peroxisome proliferator-activated receptor-gamma2 Pro12Ala (PPAR-γ2 Pro12Ala), has been reported to have a preventive role in diabetes mellitus by decreasing insulin resistance in Caucasians, but not in Asians [20,30]. The frequency of PPAR-γ2 Pro12Ala polymorphism has been shown to be considerably lower in Asians than in Caucasians [30,31]. Other possible thrifty genes that may contribute to the difference in fat and energy metabolism between Koreans and Caucasians are the uncoupling protein-3 (UCP3) gene and the intestinal fatty acid-binding protein-2 (FABP2) gene. The recessive homozygote of UPC3 gene is associated with a higher BMI, and is seen more frequently in Asians than Caucasians (48% and 22%, respectively). The UCP3 is also related to blood glucose and seems to significantly affect sugar metabolism and the onset of diabetes mellitus [31]. The intestinal FABP2 related to lipid metabolism has been associated with obesity because it enhances fat absorption. The allelic frequency of FABP2 is 55% in Asians and 27.1% in Caucasians. Thus, if Asians consume the same amount of fat, a higher body fat deposit at a lower or the same BMI would be observed in Asians [25,31] South Korea is one of the countries where the socioeconomic environment has changed rapidly to reflect more Westernization, including its negative health consequences. With the adoption of a more Westernized lifestyle, higher dietary fat consumption and less physical activity are common in Korea, and hence, these thrifty genes heighten the susceptibility to insulin insensitivity and cardiovascular diseases in relation to increased body fat and dyslipidemia in Koreans [20,25,26]. Increased body fat and dyslipidemia are associated with the induction of proinflammatory cytokines, adhesion molecules, and reactive oxygen species (ROS) within the vascular walls [32]. Increased superoxide anion radicals by leukocytes cause reduced nitric oxide bioavailability due to either reduced formation or accelerated degradation of nitric oxide, causing cell membrane injury, and induce low-density lipoprotein oxidation, which has cytotoxic effects on the vascular endothelial cells [33]. These factors, taken together, can induce endothelial dysfunction leading to an impaired skin blood flow response to occlusion and local heating. The interaction of thrifty genes and rapidly changed lifestyles may explain why Koreans have a lower skin blood flow response to occlusion and local heating due to endothelial dysfunction than Caucasians. International studies conducted among different Asian national populations in China, Korea, Philippines, Singapore, and Taiwan have shown increased risk of Type 2 diabetes and cardiovascular disease at lower BMI than European populations [28].
Previous studies show that when heat is applied to the skin, the ability of heat transfer through the skin into deeper tissues is impaired in individuals with a thinner dermal layer, thicker subcutaneous fat layer, and lower skin moisture, leading to reduced skin blood flow response in local heating [14,18,34]. For example, a recent study by Petrofsky et al. showed a 100% increase in skin blood flow when the skin was moist versus when it was dry, as well as a reduced sensitivity for changes in blood flow with local heat applications [18]. Also, subcutaneous fat impairs the ability of circulation to transfer heat into deeper tissues, which may contribute to higher skin temperatures during local heating [14,18,34]. For these reasons, in this study we measured characteristics of the skin such as skin thickness, subcutaneous fat thickness, and skin moisture. However, there was no significant difference between Koreans and Caucasians. Thus, the difference in skin blood flow may not be due to characteristics of the skin in this study.
Local heat application to the skin results in a large increase in skin blood flow that is biphasic and characterized by an initial response followed by a more prolonged response [14–17]. In the first few minutes, the initial blood flow response to local heat is primarily mediated by tactile sensors in the skin through the release of substance P and calcitonin gene-related peptide from sensory nerves. The secondary sustained blood flow response to local heat is mediated primarily by nitric oxide released from vascular endothelial cells [8,14,16–18]. Endothelial nitric oxide synthetase is activated by TRPV4 voltage gated, temperature sensitive calcium channels [8,15]. In the present study, skin blood flow response to local heat was higher in Caucasians than in Koreans after 60 seconds of heat exposure at 38°C and 40°C and after 90 seconds of heat exposure at 42°C (Figures 3–5, respectively). In other words, in the first 60 or 90 seconds of the initial blood flow response mediated by tactile neurons, there was no significant difference between Koreans and Caucasians. After the 60 or 90 seconds of the initial response, however, there was significantly higher blood flow response in Caucasians than in Koreans during the sustained blood flow response mediated by nitric oxide. Thus, this result would seem to imply that Koreans have lower vascular endothelial function than Caucasians, primarily due to the reduced bioavailability of nitric oxide, not the tactile receptors.
The results of this study may also infer greater susceptibility to skin burns in Koreans. Many studies indicated that it was both the conductive heat loss through the skin itself and skin blood flow that are important in dissipating heat from the skin [16,18,35]. However, conductive heat loss for living skin is not constant (a second or less) and the skin blood flow does play a progressively greater role in continually removing heat for protecting the skin from burns [14,16,35]. According to a recent study from this laboratory, older people and people with diabetes are more susceptible to skin damage and burns due to changes in the structures of the skin and a reduction in skin blood flow linked to reduced bioavailability of nitric oxide by free radicals in vascular endothelial cells [14]. In the present study, as mentioned before, there was no significant difference in skin thickness, subcutaneous fat thickness, and skin moisture between Koreans and Caucasians. As shown in Figures 3–5, however, Korean subjects had significantly lower blood flow response to local heating than Caucasians and it is strongly believed that Koreans are more susceptible to skin burns than Caucasians.
In this study, Korean subjects had been living in the United States (US), rather than in Korea. Although the mean period of stay in US was 7.1±2.3 months in Korean subjects, the results might be different in Koreans who did not reside in the US. In addition, due to different diets, especially high-fat diet, and environment, Koreans who have been in the US for longer periods may have more reduced endothelial function than those with shorter periods of US residence. Therefore, further studies need to be conducted to compare Koreans who have been in the US for long periods and those who have not been to the US. These results are only in 10 subjects and therefore have limited power in them-selves. However, similar results have been published for the vascular response to occlusion in people from India [36] and Thailand [20], increasing the strength of these conclusions. Seemingly contradicting these results are the results of a recent study showing that the death rates from heart disease are much lower in Korea than in the United States [37]. However, the prevalence of cardiovascular disease and diabetes in African-Americans and Asian-Americans is much higher than in non-Hispanic whites in the US, skewing the Caucasian data [28,38,39]. Also, the incidence of obesity and diabetes is increasing at a much higher rate in Koreans than in non-Hispanic whites in the US [28,29], as it is across Asia. It has only been in the last 10 years that diabetes and obesity have increased dramatically in Asia to the point where the World Health Organization (WHO) has called this an epidemic. It will be interesting to see if, due to an increase in high fat food, the death rates remain this low 10–20 years from now in Korea. It will take time to see the effects of Western diet on Asians.
Conclusions
In the present investigation, endothelial function was assessed by the blood flow response to local heat and vascular occlusion. In the reactive hyperemia after 4 minutes of vascular occlusion, the skin blood flow was significantly higher in Caucasians than Koreans. In the response to 6 minutes of local heating at 38°C, 40°C, and 42°C, the skin blood flow was significantly higher in Caucasians than Koreans. These findings suggest that the Korean population has lower vascular endothelial function than Caucasians.
Footnotes
Source of support: Departmental sources
References
- 1.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568(Pt 2):357–69. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joannides R, Bellien J, Thuillez C. Clinical methods for the evaluation of endothelial function – a focus on resistance arteries. Fundam Clin Pharmacol. 2006;20(3):311–20. doi: 10.1111/j.1472-8206.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 3.Binggeli C, Spieker LE, Corti R, et al. Statins enhance postischemic hyperemia in the skin circulation of hypercholesterolemic patients: a monitoring test of endothelial dysfunction for clinical practice? J Am Coll Cardiol. 2003;42(1):71–77. doi: 10.1016/s0735-1097(03)00505-9. [DOI] [PubMed] [Google Scholar]
- 4.Medow MS, Taneja I, Stewart JM. Cyclooxygenase and nitric oxide synthase dependence of cutaneous reactive hyperemia in humans. Am J Physiol Heart Circ Physiol. 2007;293(1):H425–32. doi: 10.1152/ajpheart.01217.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minson CT, Wong BJ. Reactive hyperemia as a test of endothelial or microvascular function? J Am Coll Cardiol. 2004;43(11):2147. doi: 10.1016/j.jacc.2004.03.005. author reply 2147–48. [DOI] [PubMed] [Google Scholar]
- 6.Wong BJ, Wilkins BW, Holowatz LA, Minson CT. Nitric oxide synthase inhibition does not alter the reactive hyperemic response in the cutaneous circulation. J Appl Physiol. 2003;95(2):504–10. doi: 10.1152/japplphysiol.00254.2003. [DOI] [PubMed] [Google Scholar]
- 7.Ciplak M, Pasche A, Heim A, et al. The vasodilatory response of skin microcirculation to local heating is subject to desensitization. Microcirculation. 2009;16(3):265–75. doi: 10.1080/10739680802595880. [DOI] [PubMed] [Google Scholar]
- 8.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol. 2010;95(9):946–54. doi: 10.1113/expphysiol.2010.053538. [DOI] [PubMed] [Google Scholar]
- 9.Minson CT, Holowatz LA, Wong BJ, et al. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93(5):1644–49. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 10.Cuevas AM, Germain AM. Diet and endothelial function. Biol Res. 2004;37(2):225–30. doi: 10.4067/s0716-97602004000200008. [DOI] [PubMed] [Google Scholar]
- 11.Deanfield J, Donald A, Ferri C, et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23(1):7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90(10C):40–48L. doi: 10.1016/s0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- 13.Petrofsky J, Lee S. The effects of type 2 diabetes and aging on vascular endothelial and autonomic function. Med Sci Monit. 2005;11(6):CR247–54. [PubMed] [Google Scholar]
- 14.Petrofsky J, Lee H, Trivedi M, et al. The Influence of Aging and Diabetes on Heat Transfer Characteristics of the Skin to a Rapidly Applied Heat Source. Diabetes Technol Ther. 2010;12(12):1003–10. doi: 10.1089/dia.2010.0152. [DOI] [PubMed] [Google Scholar]
- 15.Petrofsky J, Goraksh N, Alshammari F, et al. The ability of the skin to absorb heat; the effect of repeated exposure and age. Med Sci Monit. 2011;17(1):CR1–8. doi: 10.12659/MSM.881315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrofsky J, Paluso D, Anderson D, et al. The Ability of Different Areas of the Skin to Absorb Heat from a Locally Applied Heat Source: The Impact of Diabetes. Diabetes Technol Ther. 2011;13(3):365–72. doi: 10.1089/dia.2010.0161. [DOI] [PubMed] [Google Scholar]
- 17.Petrofsky JS, Bains G, Raju C, et al. The effect of the moisture content of a local heat source on the blood flow response of the skin. Arch Dermatol Res. 2009;301(8):581–85. doi: 10.1007/s00403-009-0957-3. [DOI] [PubMed] [Google Scholar]
- 18.Petrofsky JS, McLellan K, Bains GS, et al. Skin heat dissipation: the influence of diabetes, skin thickness, and subcutaneous fat thickness. Diabetes Technol Ther. 2008;10(6):487–93. doi: 10.1089/dia.2008.0009. [DOI] [PubMed] [Google Scholar]
- 19.McLellan K, Petrofsky JS, Zimmerman G, et al. Multiple stressors and the response of vascular endothelial cells: the effect of aging and diabetes. Diabetes Technol Ther. 2009;11(2):73–79. doi: 10.1089/dia.2008.0026. [DOI] [PubMed] [Google Scholar]
- 20.Bui C, Petrofsky J, Berk L, et al. Acute effect of a single high-fat meal on forearm blood flow, blood pressure and heart rate in healthy male Asians and Caucasians: a pilot study. Southeast Asian J Trop Med Public Health. 2010;41(2):490–500. [PMC free article] [PubMed] [Google Scholar]
- 21.Petrofsky J, Paluso D, Anderson D, et al. The contribution of skin blood flow in warming the skin after the application of local heat; the duality of the Pennes heat equation. Med Eng Phys. 2011;33(3):325–29. doi: 10.1016/j.medengphy.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Petrofsky JS. A device to measure heat flow through the skin in people with diabetes. Diabetes Technol Ther. 2010;12(9):737–43. doi: 10.1089/dia.2010.0043. [DOI] [PubMed] [Google Scholar]
- 23.Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105(5):546–49. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- 24.Avery MR, Voegeli D, Byrne CD, et al. Age and cigarette smoking are independently associated with the cutaneous vascular response to local warming. Microcirculation. 2009;16(8):725–34. doi: 10.3109/10739680903199194. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi S, Yamane K, Kamei N, et al. The effect of polymorphism in the intestinal fatty acid-binding protein 2 gene on fat metabolism is associated with gender and obesity amongst non-diabetic Japanese-Americans. Diabetes Obes Metab. 2004;6(1):45–49. doi: 10.1111/j.1463-1326.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- 26.Murphy C, Kanaganayagam GS, Jiang B, et al. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol. 2007;27(4):936–42. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- 27.Kahn DF, Duffy SJ, Tomasian D, et al. Effects of black race on forearm resistance vessel function. Hypertension. 2002;40(2):195–201. doi: 10.1161/01.hyp.0000024571.69634.ed. [DOI] [PubMed] [Google Scholar]
- 28.Palaniappan LP, Wong EC, Shin JJ, et al. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond) 2011;35(3):393–400. doi: 10.1038/ijo.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27(1):66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 30.Radha V, Vimaleswaran KS, Babu HN, et al. Role of genetic polymorphism peroxisome proliferator-activated receptor-gamma2 Pro12Ala on ethnic susceptibility to diabetes in South-Asian and Caucasian subjects: Evidence for heterogeneity. Diabetes Care. 2006;29(5):1046–51. doi: 10.2337/diacare.2951046. [DOI] [PubMed] [Google Scholar]
- 31.Kagawa Y, Yanagisawa Y, Hasegawa K, et al. Single nucleotide polymorphisms of thrifty genes for energy metabolism: evolutionary origins and prospects for intervention to prevent obesity-related diseases. Biochem Biophys Res Commun. 2002;295(2):207–22. doi: 10.1016/s0006-291x(02)00680-0. [DOI] [PubMed] [Google Scholar]
- 32.Poppitt SD, Kilmartin P, Butler P, Keogh GF. Assessment of erythrocyte phospholipid fatty acid composition as a biomarker for dietary MUFA, PUFA or saturated fatty acid intake in a controlled cross-over intervention trial. Lipids Health Dis. 2005;4:30. doi: 10.1186/1476-511X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bae JH, Bassenge E, Kim KB, et al. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis. 2001;155(2):517–23. doi: 10.1016/s0021-9150(00)00601-8. [DOI] [PubMed] [Google Scholar]
- 34.McLellan K, Petrofsky JS, Bains G, et al. The effects of skin moisture and subcutaneous fat thickness on the ability of the skin to dissipate heat in young and old subjects, with and without diabetes, at three environmental room temperatures. Med Eng Phys. 2009;31(2):165–72. doi: 10.1016/j.medengphy.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Pennes HH. Analysis of tissue and arterial blood temperatures in the resting human forearm. J Appl Physiol. 1948;1(2):93–122. doi: 10.1152/jappl.1948.1.2.93. [DOI] [PubMed] [Google Scholar]
- 36.Petrofsky JS, Alshahmmari F, Lee H, et al. Reduced endothelial function in the skin in Southeast Asians compared to. Caucasians Med Sci Monit. 2012;18(1):CR1–8. doi: 10.12659/MSM.882185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Writing Group M. Roger VL, Go AS, et al. Heart Disease and Stroke Statistics – 2012 Update: A Report From the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bransford TL, St Vrain JA, Webb M. Abnormal endothelial function in young African-American females: discordance with blood flow. J Natl Med Assoc. 2001;93(4):113–19. [PMC free article] [PubMed] [Google Scholar]
- 39.Cardillo C, Kilcoyne CM, Cannon RO, III, Panza JA. Attenuation of cyclic nucleotide-mediated smooth muscle relaxation in blacks as a cause of racial differences in vasodilator function. Circulation. 1999;99(1):90–95. doi: 10.1161/01.cir.99.1.90. [DOI] [PubMed] [Google Scholar]