Summary
Background
Exposure to a warm environment has been reported to be effective for recovery from mental fatigue. However, there have been no reports examining the effects of a pellet stove on recovery from mental fatigue. The purpose of this study was to examine the effects of a pellet stove on recovery from mental fatigue.
Material/Methods
In this placebo-controlled, crossover experiment, 16 healthy volunteers were randomized into the pellet stove and control groups. After a 30-min fatigue-inducing mental task session, participants moved to a recovery room with (pellet stove condition) or without (control condition) a pellet stove to see the image of a pellet stove for 30 min.
Results
After the recovery session, the participants exposed to the pellet stove condition showed lower total error counts of a cognitive test, higher levels of subjective healing, comfort, and warmth, and sympathetic nerve activity and higher parasympathetic nerve activity as compared with the control condition.
Conclusions
These results provide evidence that improved cognitive function, subjective mental states, and balance of the autonomic nervous activities result from using a pellet stove during the recovery session. Hence, the pellet stove was effective for the recovery from mental fatigue.
Keywords: accelerated plethysmography, advanced trail making test, mental fatigue, pellet stove, recovery, 2-back test
Background
Fatigue, best defined as difficulty in initiating or sustaining voluntary activities [1], is a common symptom of various illnesses and even occurs in healthy individuals [2–4]. In Japan, more than half of the general adult population complains of fatigue [5]. Acute fatigue is a normal phenomenon that disappears after a period of rest. In contrast, long-term fatigue (chronic fatigue) is sometimes irreversible, and the compensation mechanisms that are useful in reducing acute fatigue are ineffective. Chronic fatigue is thought to be caused by prolonged accumulation of acute fatigue. Thus, to avoid chronic fatigue, it is important to develop effective strategies to recover from and avoid accumulation of acute fatigue.
Positive effects of hot-water bathing in micro-bubbles on recovery from mental fatigue have been reported [6]. In addition, a warm-water footbath can relieve fatigue, probably because the footbath promotes circulation and removes metabolites such as radical oxygen species, resulting in relief of fatigue [7]. However, it is often difficult to spend a great deal of time under these warm conditions. Using heating apparatuses such as a stove, we can easily spend long periods exposed to a warm environment in the cold seasons, which may help in the recovery from mental fatigue. In particular, a pellet stove, which burns compressed wood or biomass pellets, is ecological and generally believed to increase core temperature effectively though its “far infrared ray effect.” Hypothermia has been shown to decrease cerebral blood flow, and cold conditions lead to deterioration of autoregulation of cerebral blood flow. Thus, the pellet stove might increase core temperature resulting in the promotion of the recovery of the inactivated cerebral blood flow caused by mental fatigue. Since mental fatigue impairs our daily activities, it is of great value to develop effective methods to promote recovery from mental fatigue.
The aim of this study was to test the effects of a pellet stove on recovery from mental fatigue using recently established fatigue-inducing and evaluation methods [6,11]. After a mental fatigue-inducing task, participants moved to a room equipped with or without a pellet stove. We measured mental task performance, various subjective sensations, and autonomic nervous system activities immediately before and after a recovery session from mental fatigue to evaluate the fatigue-recovering effects of spending timing in a room equipped with a pellet stove.
Material and Methods
Participants
Sixteen healthy volunteers (28.4±7.2 years of age [mean±SD]); seven females and nine males] were enrolled in this randomized, placebo-controlled, crossover experiment (control and pellet stove conditions). Current smokers, individuals having history of medical illness, taking chronic medication, or supplemental vitamins were excluded. The study protocol was approved by the Ethics Committee of Osaka City University, and all the participants provided written informed consent for participation in the study.
Experimental design
This study was performed between March 16, 2010, and March 24, 2010. After enrollment, participants were randomly assigned to two groups in a single-blinded, crossover fashion to perform fatigue-inducing and recovery sessions. As a fatigue-inducing mental task, they performed a 2-back test [12] for 30 min [13], and as fatigue-evaluating mental tasks, they performed advanced trail making tests (ATMTs) [14] for 20 min just before and after the fatigue-inducing session. They were asked to rate their levels of fatigue, healing, comfort, and warmth on a visual analogue scale (VAS) from 0 (minimum) to 100 (maximum) to evaluate their subjective mental state [15] before and after the fatigue-inducing task. Participants also underwent accelerated plethysmography (APG) before and after the fatigue-inducing task. Then they moved to a recovery room. During the recovery session, they sat on a floor quietly for 30 min in a room equipped with either a pellet stove (MODANRO; Kondo Tekko Co., Ltd., Nagano, Japan) (pellet stove condition) or a monitor on which a pellet stove was displayed (control condition). After the recovery session, they were again subjected to the evaluation of ATMTs, VAS, and APG. The study was conducted in a quiet temperature- and humidity-controlled environment, and the temperature and humidity were similar between the pellet and control stove conditions. For 1 day before each visit, participants refrained from intense mental and physical activity, consumed a normal diet and beverages, and maintained normal sleeping hours. The time interval between each experiment was 3–5 days.
Fatigue-inducing task
As a fatigue-inducing task, participants performed a 2-back test for 30 min. Either of four types of letters was continually presented on a display of a personal computer every 3 sec. Participants had to judge whether the target letter presented at the center of the screen was the same as the one that had appeared two presentations before. If it was, they were to click the right mouse button with their right middle finger; if it was not, they were to click the left mouse button. They were instructed to perform the task trials as quickly and as correctly as possible. The result of the 2-back trial – correct response or error – was continually presented on the display of the personal computer.
Fatigue-evaluating tasks
As fatigue-evaluation tasks, participants performed ATMTs for 20 min. During these tests, circles numbered from 1 to 25 were randomly located on the display of a personal computer, and participants were required to click these circles in sequence using a computer mouse, starting with circle number 1. When they clicked the 25th target, the ATMT was considered completed. In ATMT task A, when they clicked a target circle, it remained at the same position, but the color of the circle changed from black to yellow. The positions of other circles remained the same. In ATMT task B, when they clicked the first target circle, it disappeared, and circle number 26 appeared in a different position on the screen. The positions of other circles remained the same. Subsequently, clicking, for example, circles 2, 3, and 4 resulted in these particular circles disappearing and the addition of circles 27, 28, and 29 on the screen, so that the number of circles seen on the screen was always 25. In ATMT task C, when participants clicked the first target circle, it disappeared and circle number 26 appeared in a different position on the screen, and the positions of all other circles were changed at random. Subsequently, clicking, for example, circles 2, 3, and 4 resulted in these particular circles disappearing and the addition of circles 27, 28, and 29 on the screen, so that the number of circles seen on the screen was always 25. They performed tasks A, B, and C consecutively in this order. They were instructed to perform these task trials as quickly and as correctly as possible.
APG
APG has been used for the evaluation of autonomic activities [6,11,16,17]. In the present study, APG was performed using a pulsimeter (Artett, U-Medica, Osaka, Japan) with the sensor positioned on the tip of the ventral side of the index finger. Photoplethysmography was used to measure changes in the absorption of light by hemoglobin, which is related to blood flow volume. The pulsimeter performed automatic analyses of the second derivative of the photoplethysmographic waveform, which is known as the APG waveform. Participants underwent APG sitting quietly with their eyes closed for 1 min. The APG waveform consists of four waves in systole (a–d) and one in diastole (e). Sensor output of the pulsimeter was preprocessed by a second-order analog low-pass filter with 23 Hz of cut off frequency. Data were recorded (3.3 volts to 10 bits) using an analog-to-digital converter and real-time at a sampling rate of 1,000 samples per second. These digital data were processed with the 67th order, finite impulse-response filter using the Hanning window. Detected peak times were interpolated to sub-millisecond order. Frequency analyses for pulse-interval variation were analyzed with fast Fourier transformation. The resolution ability for the power spectrum was 0.001 Hz. For the frequency analyses, the total power was calculated as the power within a frequency range of 0–0.4 Hz, the low-frequency component power (LF) was calculated as the power within a frequency range of 0.04–0.15 Hz, and the high-frequency component power (HF) was calculated as that within a frequency range of 0.15–0.4 Hz. LF and HF were assessed in normalized units; normalization was performed by dividing the absolute power of each component by the total variance and then multiplying by 100. The %HF is vagally mediated [18–20], whereas %LF originates from a variety of sympathetic and vagal mechanisms [18,21]. LF/HF ratio is considered to represent sympathetic activity [21].
Statistical analysis
The paired t-test was used to evaluate the significance of differences between the control and pellet stove conditions. To control for intraindividual variability of baseline values before the recovery session, changes from values before the recovery session to those after the recovery session, were calculated. All P values were 2-tailed, and those less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS 17.0J (SPSS Inc, Chicago, IL, USA).
Results
Subjective parameters before and after the fatigue-inducing sessions and after the recovery session are shown in Table 1. VAS scores for fatigue, healing, comfort, and warmth before and after the fatigue-inducing sessions did not differ significantly between the control and pellet stove conditions. After the recovery session, subjective scores for fatigue were significantly lower in the pellet stove condition compared with the control condition, and values for comfort and warmth were significantly higher than those in the control condition. Values for healing trended toward significance between groups. Changes of subjective scores for comfort and warmth in the pellet stove condition were significantly higher than those in the control condition and changes in values for healing trended toward significance (Table 2).
Table 1.
Subjective parameters before and after fatigue-inducing sessions and after recovery session as assessed using a visual analogue scale from 0 (minimum) to 100 (maximum).
| Control | Pellet stove | p value | |
|---|---|---|---|
| Fatigue | |||
| Before fatigue | 26.2±26.0 | 16.1±17.2 | 0.166 |
| After fatigue | 47.1±30.8 | 39.3±26.4 | 0.215 |
| After recovery | 25.3±14.7 | 15.9±13.9 | 0.047 |
| Healing | |||
| Before fatigue | 27.4±20.5 | 32.0±25.0 | 0.131 |
| After fatigue | 14.9±16.7 | 15.3±16.6 | 0.980 |
| After recovery | 39.9±20.0 | 50.5±23.0 | 0.068 |
| Comfort | |||
| Before fatigue | 36.7±17.6 | 29.1±21.7 | 0.174 |
| After fatigue | 19.1±18.9 | 18.1±18.6 | 0.576 |
| After recovery | 35.7±20.7 | 47.0±28.5 | 0.043 |
| Warmth | |||
| Before fatigue | 31.1±23.3 | 32.7±17.2 | 0.587 |
| After fatigue | 23.5±20.3 | 23.4±16.9 | 0.731 |
| After recovery | 35.6±22.9 | 56.5±20.7 | 0.006 |
Data are presented as mean ±SD.
Table 2.
Changes in subjective parameters after the recovery session.
| Control | Pellet stove | p value | |
|---|---|---|---|
| Fatigue | −21.9±19.7 | −23.4±26.8 | 0.310 |
| Healing | 25.1±20.9 | 35.2±29.1 | 0.082 |
| Comfort | 16.5±22.4 | 28.9±30.9 | 0.024 |
| Warmth | 12.1±23.4 | 33.1±29.5 | 0.001 |
Data are presented as mean±SD.
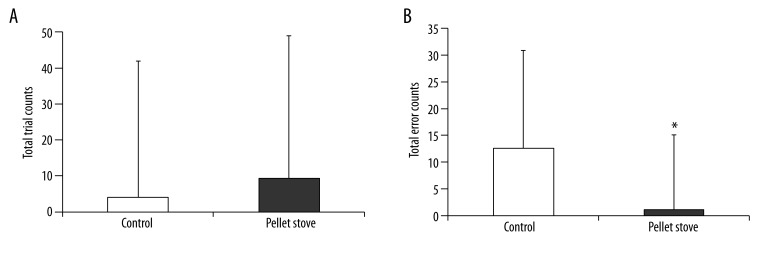
Cognitive task parameters before and after the fatigue-inducing sessions and after the recovery session are shown in Table 3, and changes of the performances of the cognitive tasks after the recovery session are shown in Figure 1. Although changes in total trial counts after the recovery session did not differ between the control and pellet stove conditions, changes in total error counts in the pellet stove condition were significantly lower than that in the control condition.
Table 3.
Cognitive task and autonomic nervous parameters before and after fatigue-inducing sessions and after recovery session.
| Control | Pellet stove | p value | |
|---|---|---|---|
| Total trial counts | |||
| Before fatigue | 587.5±121.3 | 587.9±80.3 | 0.591 |
| After fatigue | 584.2±119.3 | 599.5±87.8 | 0.525 |
| After recovery | 588.3±111.6 | 608.7±93.6 | 0.419 |
| Total error counts | |||
| Before fatigue | 31.5±27.0 | 37.4±45.6 | 0.270 |
| After fatigue | 36.9±25.9 | 43.7±42.4 | 0.238 |
| After recovery | 49.5±38.6 | 44.9±43.6 | 0.535 |
| LF/HF ratio | |||
| Before fatigue | 1.8±1.9 | 3.7±5.8 | 0.097 |
| After fatigue | 1.7±1.2 | 3.3±4.6 | 0.115 |
| After recovery | 2.5±2.1 | 1.0±0.5 | 0.016 |
| %HF | |||
| Before fatigue | 47.7±19.7 | 38.6±21.4 | 0.083 |
| After fatigue | 47.0±21.7 | 39.5±21.6 | 0.039 |
| After recovery | 40.4±21.6 | 52.6±12.8 | 0.069 |
LF – low-frequency component; HF – high-frequency component. Data are presented as mean±SD.
Figure 1.
Changes of total trial counts (A) and total error counts (B) of advanced trail making tests after the recovery session. Data are presented as mean and SD. * p<0.05, significant difference between control and pellet stove conditions (paired t-test).
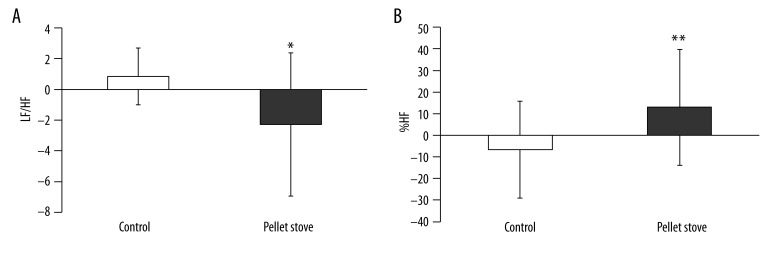
APG parameters before and after the fatigue-inducing sessions and after the recovery session are shown in Table 3, and changes of the APG parameters after the recovery session are shown in Figure 2. Changes in the sympathetic nerve activity after the recovery session in the pellet stove condition were significantly lower than those in the control condition; and changes of parasympathetic nerve activity in the pellet stove condition were significantly higher than that in the control condition.
Figure 2.
Changes of low-frequency component (LF)/high-frequency component (HF) ratio (A) and %HF (B) after the recovery session. Data are presented as mean ±SD. ** p<0.01, * p<0.05, significant difference between control and pellet stove conditions (paired t-test).
Discussion
We found that mental task performance after the recovery session in the pellet stove condition was better than that in the control condition. In addition, higher levels of subjective healing, comfort, and warmth, and lower sympathetic nerve activity and higherpara sympathetic nerve activity were seen in the pellet stove condition compared with the control condition. These results provide evidence that improved cognitive function, subjective mental states, and balance of the autonomic nervous system activities resulted from using a pellet stove during a period of recovery from mental fatigue. Our results are consistent with the results of a previous report of hot-water bathing in micro-bubbles; although temperature of the water was similar between the bathing in micro-bubbles and the normal bathing, subjective level of warmth was higher in the micro-bubble-bathing condition, and simultaneously this bathing condition showed positive effects on recovery from mental fatigue [6].
Previously, we showed that participants were fatigued by performing 30-min 2-back test trials through the evaluations of the task performance of ATMTs just before and after the mental fatigue-inducing session; after the mental fatigue-inducing session, the total error counts of the cognitive tests increased [23]. Because the performance of the cognitive tasks is thought to require selective attention [13], the decreased total error counts after the recovery session in the pellet stove condition might be caused by an increase in the ability to maintain selective attention.
The brain network, including the prefrontal cortex (PFC) and anterior cingulate cortex (ACC), has been shown to play an important role in the regulation of autonomic nervous system activities [24]. Decreased parasympathetic activity and increased sympathetic activity are interpreted as a state of autonomic hypervigilance [25,26], and sympathoexcitatory subcortical circuits are normally under the inhibitory control of the PFC [25–27]. In addition, the ACC is related to the regulation of parasympathetic activity [28,29]. Because impaired selective attention assessed by increased error counts of the ATMTs [13] was observed after the fatigue-inducing task, and the selective attention process activates the PFC and ACC [30–33], acute mental load might introduce temporary dysfunctions in the PFC and ACC to cause decreased parasympathetic and increased sympathetic activities as well as inability to use selective attention. Considering that the pellet stove condition showed an increased ability in participants to use selective attention and increased parasympathetic and decreased sympathetic activities, the pellet stove may be effective for recovery from mental fatigue through the activation of the PFC and ACC.
The mechanism by which the pellet stove was effective for the recovery from mental fatigue is not clear. However, subjective level of warmth was higher in the pellet stove condition although temperature of the rooms was similar between the pellet stove and the control condition. Hypothermia has been shown to decrease cerebral blood flow [8,9], and cold conditions lead to deterioration of autoregulation of cerebral blood flow [10]. Thus, one possible mechanism for the effects seen in this study is that the pellet stove increased core temperature resulting in the promotion of the recovery of the inactivated cerebral blood flow and function in the PFC and ACC.
There are two potential limitations in this study. First, we conducted this study with a limited number of participants. To generalize our results, studies involving a larger number of participants are essential. Second, we did not measure core temperature of participants. Further studies are necessary to clarify the mechanisms of the pellet stove to accelerate the recovery from mental fatigue.
Conclusions
In conclusion, we demonstrated that the pellet stove was effective for recovery from mental fatigue. To avoid chronic fatigue, it is important to develop effective strategies to attenuate and recover from acute mental fatigue. Use of a pellet stove might prevent unfavorable situations as a result of accumulated acute mental fatigue.
Acknowledgements
We thank Forte Science Communication for editorial assistance with the manuscript.
Footnotes
Source of support: This work was supported in part by the Grant-in-Aid for Scientific Research B (KAKENHI: 23300241) from Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan
References
- 1.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–88. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 2.Grandjean EP. Fatigue. Am Ind Hyg Assoc J. 1970;31:401–11. doi: 10.1080/0002889708506267. [DOI] [PubMed] [Google Scholar]
- 3.Ream E, Richardson A. Fatigue: a concept analysis. Int J Nurs Stud. 1996;33:519–29. doi: 10.1016/0020-7489(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 4.Ream E, Richardson A. Fatigue in patients with cancer and chronic obstructive airways disease: a phenomenological enquiry. Int J Nurs Stud. 1997;34:44–53. doi: 10.1016/s0020-7489(96)00032-6. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe Y. Preface and mini-review: fatigue science for human health. In: Watanabe Y, Evengård B, Natelson BH, et al., editors. Fatigue Science for Human Health. 1st ed. New York: Springer; 2008. pp. 5–11. [Google Scholar]
- 6.Tajima K, Tanaka M, Mizuno K, et al. Effects of bathing in micro-bubbles on recovery from moderate mental fatigue. Ergonomia IJE&HF. 2008;30:135–45. [Google Scholar]
- 7.Yang HL, Chen XP, Lee KC, et al. The effects of warm-water footbath on relieving fatigue and insomnia of the gynecologic cancer patients on chemotherapy. Cancer Nurs. 2010;33:454–60. doi: 10.1097/NCC.0b013e3181d761c1. [DOI] [PubMed] [Google Scholar]
- 8.Soukup J, Zauner A, Doppenberg EM, et al. The importance of brain temperature in patients after severe head injury: relationship to intracranial pressure, cerebral perfusion pressure, cerebral blood flow, and outcome. J Neurotrauma. 2002;19:559–71. doi: 10.1089/089771502753754046. [DOI] [PubMed] [Google Scholar]
- 9.Sahuquillo J, Vilalta A. Cooling the injured brain: how does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des. 2007;13:2310–22. doi: 10.2174/138161207781368756. [DOI] [PubMed] [Google Scholar]
- 10.Doering TJ, Aaslid R, Steuernagel B, et al. Cerebral autoregulation during whole-body hypothermia and hyperthermia stimulus. Am J Phys Med Rehabil. 1999;78:33–38. doi: 10.1097/00002060-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Mizuno K, Tanaka M, Tajima K, et al. Effects of mild-stream bathing on recovery from mental fatigue. Med Sci Monit. 2010;16(1):8–14. [PubMed] [Google Scholar]
- 12.Braver TS, Cohen JD, Nystrom LE, et al. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno K, Watanabe Y. Utility of an advanced trail making test as a neuropsychological tool for an objective evaluation of work efficiency during mental fatigue. In: Watanabe Y, Evengård B, Natelson BH, et al., editors. Fatigue Science for Human Health. 1st ed. New York: Springer; 2008. pp. 47–54. [Google Scholar]
- 14.Kajimoto O. Development of a method of evaluation of fatigue and its economic impacts. In: Watanabe Y, Evengård B, Natelson BH, et al., editors. Fatigue Science for Human Health. 1st ed. New York: Springer; 2008. pp. 33–46. [Google Scholar]
- 15.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36:291–98. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguti K. The evaluation of fatigue by using acceleration plethysmography. Nippon Rinsho. 2007;65:1034–42. [PubMed] [Google Scholar]
- 17.Takada M, Ebara T, Kamijima M. Heart rate variability assessment in Japanese workers recovered from depressive disorders resulting from job stress: measurements in the workplace. Int Arch Occup Environ Health. 2010;83:521–29. doi: 10.1007/s00420-009-0499-1. [DOI] [PubMed] [Google Scholar]
- 18.Akselrod S, Gordon D, Ubel FA, et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–22. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 19.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248:151–53. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 20.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–92. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 21.Appel ML, Berger RD, Saul JP, et al. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol. 1989;14:1139–48. doi: 10.1016/0735-1097(89)90408-7. [DOI] [PubMed] [Google Scholar]
- 22.Pagani M, Montano N, Porta A, et al. Relationship between spectral components of cardiovascular variabilities and direct measures of muscle sympathetic nerve activity in humans. Circulation. 1997;95:1441–48. doi: 10.1161/01.cir.95.6.1441. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, Mizuno K, Tajima S, et al. Central nervous system fatigue alters autonomic nerve activity. Life Sci. 2009;84:235–39. doi: 10.1016/j.lfs.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Tang YY, Ma Y, Fan Y, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci USA. 2009;106:8865–70. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thayer JF. On the importance of inhibition: central and peripheral manifestations of nonlinear inhibitory processes in neural systems. Dose Response. 2006;4:2–21. doi: 10.2203/dose-response.004.01.002.Thayer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–72. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 27.Amat J, Baratta MV, Paul E, et al. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 28.Kubota Y, Sato W, Toichi M, et al. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Brain Res Cogn Brain Res. 2001;11:281–87. doi: 10.1016/s0926-6410(00)00086-0. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi M, Arito H. Effects of single and repeated cognitive tasks on autonomic balance as observed by an analysis of R-R intervals. Eur J Appl Physiol Occup Physiol. 1996;72:316–22. doi: 10.1007/BF00599691. [DOI] [PubMed] [Google Scholar]
- 30.Danckert J, Maruff P, Ymer C, et al. Goal-directed selective attention and response competition monitoring: evidence from unilateral parietal and anterior cingulate lesions. Neuropsychology. 2000;14:16–28. doi: 10.1037//0894-4105.14.1.16. [DOI] [PubMed] [Google Scholar]
- 31.Weissman DH, Giesbrecht B, Song AW, et al. Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage. 2003;19:1361–68. doi: 10.1016/s1053-8119(03)00167-8. [DOI] [PubMed] [Google Scholar]
- 32.Schreppel TJ, Pauli P, Ellgring H, et al. The impact of prefrontal cortex for selective attention in a visual working memory task. Int J Neurosci. 2008;118:1673–88. doi: 10.1080/00207450601067356. [DOI] [PubMed] [Google Scholar]
- 33.Morishima Y, Akaishi R, Yamada Y, et al. Task-specific signal transmission from prefrontal cortex in visual selective attention. Nat Neurosci. 2009;12:85–91. doi: 10.1038/nn.2237. [DOI] [PubMed] [Google Scholar]