Summary
Background
Global longitudinal peak strain (GLPS) quantifies left ventricle (LV) long-axis contractility. Early detection of LV systolic dysfunction is pivotal in diagnosis and treatment of patients with aortic stenosis (AS). This study was performed to assess LV longitudinal systolic function by GLPS derived from 2-dimensional speckle tracking imaging (2D-STI) in AS patients in comparison to standard echocardiographic parameters.
Material/Methods
Laboratory tests, standard echocardiography, tissue Doppler imaging (TDI) and 2D-STI examinations with GLPS calculation were performed in 49 consecutive patients with moderate to severe AS with LV ejection fraction ≥50% and 18 controls.
Results
While LVEF do not differentiate AS patients from controls, GLPS was significantly decreased in the AS group (−15.30±3.25% vs. −19.60±2.46% in controls, p<0.001). GLPS was significantly reduced in symptomatic AS patients as compared to the asymptomatic AS group [−15.5 (11.8–16.8) vs. −17.5 (14.7–18.9)%, p=0.02].
Conclusions
In aortic stenosis patients, despite normal left ventricle ejection fraction, long-axis left ventricular function is impaired, which manifests in global longitudinal peak strain reduction. GLPS reveals that LV function impairment is more pronounced in symptomatic as compared to asymptomatic AS patients. Further studies are needed to determine the prognostic significance of early LV function impairment in aortic stenosis patients showed by GLPS.
Keywords: aortic stenosis, speckle tracking echocardiography, global longitudinal peak strain, left ventricle function
Background
Aortic stenosis (AS) is the most common acquired heart defect, present in 2% of people over 75 years old and in 8% of those over 85 years old [1,2]. Patients with AS remain asymptomatic over a long period [3,4]. The prognosis in the asymptomatic AS period is much better than in the symptomatic, but there is still a 1% risk of sudden cardiac death despite the lack of symptoms [3,4]. Deterioration of left ventricle (LV) systolic function is a predictor of poor outcome after aortic valve replacement (AVR) [5]. However, the traditional method commonly used to assess LV systolic function – the ejection fraction (EF) – seems to lack sufficient precision. The myocardial strain and strain-rate analysis is an echocardiographic technique that enables the detection of mild LV systolic disfunction. Non-Doppler 2-dimensional speckle tracking imaging (2D-STI) is an angle-independent method showing the deformation of LV muscle in 3 directions: longitudinal, circumferential and radial [6–8].
According to the guidelines, patients with symptomatic severe AS should be scheduled for AVR. Asymptomatic patients with severe AS should be treated surgically when left ventricle systolic function is impaired (LVEF <50%) or there is an abnormal exercise test result revealing symptoms (indications in IC class) [9]. However, LVEF can be preserved in AS for a long time, in spite of the deterioration of LV systolic function. Thus, more sensitive methods should be used for the assessment of LV function.
This study was performed to assess LV longitudinal systolic function by means of global longitudinal peak strain (GLPS) examination in AS patients compared to standard echocardiographic parameters.
Material and Methods
Patients
This study was performed in 49 consecutive patients with moderate or severe AS, admitted to the Cardiac and Vascular Diseases Department, Jagiellonian University Medical College, Cracow, Poland. Moderate AS was defined as aortic valve area (AVA) 1.0–1.5 cm2, and severe AS as AVA <1.0 cm2. The control group consisted of 18 patients referred to our Department. Exclusion criteria for study group and control group were as follows: unstable coronary artery disease (CAD), impaired left ventricular systolic function defined as LVEF <50%, regional LV wall motion abnormalities, concomitant moderate or severe valvular disease, atrial fibrillation, and severe chronic renal or hepatic injury.
All patients underwent routine physical examination, blood analysis and transthoracic echocardiography. Medical histories were taken, including the presence of AS symptoms (chest pain, dyspnea, syncope). The study was performed in accordance with the actual version of the Helsinki Declaration. All patients gave their informed consent for participation in the study.
Echocardiography
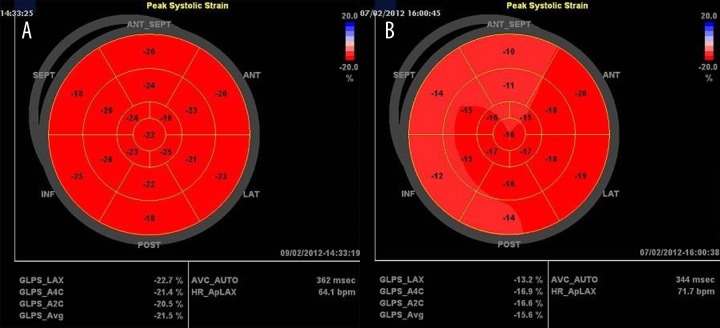
Echocardiographic examinations were performed using Vivid Seven GE Medical Systems equipment (Horton, Norway). Offline analysis was performed on the EchoPAC version 108.1.12 (GE Healthcare). Standard measurements were made in parasternal long and short axes, as well as in 4- and 5-chamber apical views. LV end-systolic, end-diastolic volume and ejection fraction were measured by the modified bi-plane Simpson method, and LV mass was calculated using the ASE formula [10]. The mean and the peak transaortic gradients were measured by continuous wave Doppler method. Aortic valve opening area (AVA) was calculated using the standard continuity equation [11]. LV mass, left atrial volume and AVA were indexed to body surface area. Peak early velocity of mitral inflow (E) was obtained using the pulsed-wave Doppler method. Peak systolic (S′) and peak early diastolic (E′) mitral annular velocities were measured at the lateral side of the mitral annulus by means of pulsed wave tissue Doppler imaging (TDI). E/E′ ratio was calculated. LV longitudinal strain was measured using 2-D speckle tracking imaging and automated function imaging (AFI) application. The ECG-gated images were obtained in apical long-axis, 4- and 2- chamber views at the frame rate of 60–70 per second, stored digitally and analyzed offline. The endocardial border of the myocardium was automatically traced and corrected manually, if needed. Segmental strains were determined offline and presented as a bulls-eye map. GLPS was automatically calculated as the mean value obtained from 17 segments (Figure 1).
Figure 1.
Segmental strain and average global longitudinal peak strain (GLPS_Avg) in a 17-segmental model presented as a “bull-eye” in control group subject (A) and in a symptomatic aortic stenosis patient (B).
Statistical analysis
Statistical analysis was performed using STATISTICA 9.0 software. The Shapiro-Wilk test was used to determine normal distribution. Continuous variables are shown as mean ± standard deviation (SD) or interquartile range [IQR]. Categorical variables are presented as number of patients and percentage. Comparisons between the groups were made by t-test for continuous variables and χ2 test for categorical variables. The Spearman correlation was used to examine linear correlation between the numerical variables. The value of p<0.05 was considered statistically significant.
Results
The demographic and clinical characteristics of aortic stenosis patients and control subjects are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the patients with aortic stenosis and in control subjects.
| Patients with aortic stenosis (n=49) | Control group (n=18) | p | |
|---|---|---|---|
| Age (years) | 68 (60–78) | 52 (31–63) | <0.001 |
| Males, n (%) | 28 (57) | 8 (44) | 0.36 |
| Smoking, n (%) | 7 (14) | 6 (35) | 0.06 |
| Hypertension, n (%) | 38 (78) | 6 (35) | <0.01 |
| Diabetes, n (%) | 11 (22) | 3 (18) | 0.94 |
| Coronary artery disease, n (%) | 20 (41) | 5 (29) | 0.40 |
By definition, AS patients had higher peak and mean transvalvular pressure gradients, with lower AVA and AVAI as compared to controls. Left ventricle mass and mass index were higher in AS patients as compared to controls (Table 2). Interestingly, although the LV ejection fraction did not significantly differ between groups, the GLPS was reduced in AS patients (−15.30±3.25% vs. −19.60±2.46%, p<0.001). Moreover, S′ and E′ velocities were reduced in the AS group, with E/E′ ratio increased in AS patients (Table 2). Figure 1 shows an example of normal GLPS recorded in a control subject and reduced GLPS obtained in an aortic stenosis patient.
Table 2.
Echocardiographic parameters in patients with aortic stenosis and in control subjects.
| Patients with aortic stenosis (n=49) | Control group (n=18) | p | |
|---|---|---|---|
| Global longitudinal peak strain (GLPS) [-%] | 15.30 (±3.25) | 19.60 (±2.46) | <0.001 |
| Ejection fraction (EF) [%] | 61.53 (±6.55) | 63.11 (±4.17) | 0.34 |
| Left ventricle mass [g] | 258 (215–339) | 186 (135–216) | <0.001 |
| Left ventricle mass index [g/m2] | 145 (122–185) | 93 (84–111) | <0.001 |
| Peak aortic gradient [mmHg] | 68.16 (±28.30) | 6.61 (±2.34) | <0.001 |
| Mean aortic gradient [mmHg] | 41.04 (±17.81) | 3.29 (±0.95) | <0.001 |
| Aortic valve area (AVA) [cm2] | 0.860 (0.710–1.200) | 2.850 (2.600–3.100) | <0.001 |
| Indexed aortic valve area (AVAI) [cm2/m2] | 0.510 (0.386–0.651) | 1.588 (1.462–1.791) | <0.001 |
| Elarly velocity of mitral inflow -E [m/s] | 0.689 (±0.251) | 0.768 (±0.221) | 0.25 |
| E/E′ ratio | 9.09 (7.00–14.50) | 6.11 (5.42–8.63) | <0.01 |
| Systolic peak annular velocity (S′) [m/s] | 0.07 (0.06–0.08) | 0.08 (0.07–0.11) | 0.02 |
| Early diastolic annular velocity (E′) [m/s] | 0.07 (0.05–0.09) | 0.12 (0.08–0.15) | <0.001 |
Among AS patients, 29 were symptomatic and 19 asymptomatic. The symptoms of 1 patient were not typical for AS, and he was excluded from the analysis. Subgroups did not differ significantly with regard to their demographics or clinical status, despite AS symptoms (Table 3).
Table 3.
Demographic and clinical characteristics of the patients with asymptomatic aortic stenosis vs. those with symptomatic aortic stenosis.
| Asymptomatic aortic stenosis (n=19) | Symptomatic aortic stenosis (n=29) | p | |
|---|---|---|---|
| Age | 68 (49–79) | 70 (62–78) | 0.35 |
| Males, n (%) | 7 (37) | 20 (69) | 0.03 |
| Smoking, n (%) | 3 (16) | 4 (14) | 0.82 |
| Hypertension, n (%) | 13 (68) | 24 (83) | 0.25 |
| Diabetes, n (%) | 4 (21) | 6 (21) | 0.74 |
| Coronary artery disease, n (%) | 5 (26) | 14 (48) | 0.13 |
| hs C-reactive protein [mg/l] | 1.20 (0.92–2.49) | 1.37 (0.61–2.31) | 0.81 |
| Total cholesterol [mmol/l] | 4.84 (±1.30) | 4.97 (±1.04) | 0.69 |
| LDL cholesterol [mmol/l] | 2.85 (±1.02) | 3.03 (±1.04) | 0.56 |
| HDL cholesterol [mmol/l] | 1.57 (±0.29) | 1.55 (±0.33) | 0.82 |
| Glucose [mmol/l] | 5.65 (5.40–6.80) | 5.50 (5.30–5.80) | 0.36 |
| Triglycerides [mmol/l] | 0.93 (0.79–1.36) | 1.11 (0.84–1.36) | 0.80 |
| Creatinine [μmol/l] | 79 (74–86) | 80 (76–94) | 0.46 |
| Alanine transaminase (ALT) [U/L] | 22 (16–28) | 25 (17–30) | 0.55 |
hs – high sensitivity.
Symptomatic patients had significantly higher transvalvular peak and mean pressure gradients and a lower aortic valve area. Importantly, GLPS and E′ velocity were significantly lower in the symptomatic patients as compared to the asymptomatic group [−15.5 (11.8–16.8) vs. −17.5 (14.7–18.9)%, p=0.02 and 0.06 (0.04–0.08) vs. 0.08 (0.06–0.11) m/s, p=0.02, respectively]. S′ velocity was insignificantly lower in the symptomatic subgroup (Table 4). Left ventricle ejection fraction, LV volumes and masses did not differ between symptomatic and asymptomatic patients.
Table 4.
Echocardiographic parameters in the patients with asymptomatic aortic stenosis vs. in those with symptomatic aortic stenosis.
| Asymptomatic aortic stenosis (n=19) | Symptomatic aortic stenosis (n=29) | p | |
|---|---|---|---|
| Global longitudinal peak strain (GLPS) [-%] | 17.5 (14.7–18.9) | 15.5 (11.8–16.8) | 0.02 |
| Ejection fraction (EF) [%] | 61.63 (±4.14) | 61.28 (±7.84) | 0.84 |
| Left ventricle end-diastolic volume (LVEDV) [ml] | 115 (81–143) | 133 (104–157) | 0.17 |
| Left ventricle end-systolic volume (LVESV) [ml] | 47 (43–70) | 56 (40–73) | 0.78 |
| Left ventricular mass [g] | 249 (178–323) | 276 (252–349) | 0.05 |
| Left ventricular mass index [g/m2] | 141 (101–158) | 167 (131–196) | 0.04 |
| Left atrial area (LAA) [cm2] | 20.44 (±5.19) | 23.86 (±5.93) | 0.05 |
| Left atrial volume (LAV) [ml] | 62.20 (±23.40) | 73.76 (±29.38) | 0.16 |
| Aorta ascending [mm] | 36.26 (±6.26) | 36.17 (±4.75) | 0.95 |
| Peak aortic gradient [mmHg] | 53 (38–66) | 71 (62–93) | 0.01 |
| Mean aortic gradient [mmHg] | 30 (21–43) | 45 (35–56) | 0.004 |
| Aortic valve area (AVA) [cm2] | 1.10 (0.85–1.30) | 0.75 (0.64–0.92) | 0.004 |
| Indexed aortic valve area (AVAI) [cm2/m2] | 0.608 (0.486–0.739) | 0.404 (0.373–0.651) | 0.07 |
| Elary velocity of mitral inflow (E) [m/s] | 0.77 (±0.26) | 0.63 (±0.24) | 0.06 |
| E/E′ | 8.89 (6.43–14.50) | 9.80 (7.17–14.67) | 0.48 |
| Systolic peak annular velocity (S′) [m/s] | 0.07 (0.06–0.08) | 0.06 (0.05–0.08) | 0.06 |
| Early diastolic annular velocity (E′) [m/s] | 0.08 (0.06–0.11) | 0.06 (0.04–0.08) | 0.02 |
While there was no correlation between GLPS and left ventricle ejection fraction, GLPS correlated positively with S′ and E′ velocities (r=0.41, p=0.004 in both cases).
Discussion
We demonstrated that LV long-axis systolic function is impaired in AS patients despite normal left ventricle ejection fraction. Aortic stenosis leads, as an adaptation to the long period of chronic pressure overload, to LV concentric hypertrophy [1,2,12]. However, increased myocardial mass results in a higher request for oxygen. Marcus et al. found decreased coronary reserve in AS subjects [13]. The disproportion between the request and the supply of the oxygen leads to ischemia and fibrosis. The most affected region of the myocardium is the subendocardial layer, where longitudinally directed fibres dominate. Interstitial fibrosis is one of the mechanisms of LV diastolic dysfunction [14]. Weidemann et al. [15] demonstrated reduction of the longitudinal strain in patients with advanced fibrosis. This may be an important factor worsening the outcome of patients after aortic valve replacement.
2D-STI is a new modality that enables quantification of myocardial deformation. The most important advantage of this technique compared to TDI is its angle independency. It enables offline measurements of the longitudinal, radial and circumferential strain and strain rate [6–8]. Because of the high sensitivity of this method, it is used to detect mild LV function abnormalities. It was shown that segmental and global longitudinal strain is decreased in coronary artery disease [16], hypertrophic cardiomyopathy [17] and dilated cardiomyopathy [18].
The Doppler-derived analysis of the segmental longitudinal, radial and circumferential strain is time consuming and requires advanced medical staff. In contrast, GLPS is an easy to obtain parameter that may be helpful in everyday clinical practice. Marwick et al. [19] examined 250 healthy volunteers (44% men, age 51±12 years) to assess the normal range of average peak systolic strain, showing no influence of age, and only mild influence of blood pressure and heart rate on average longitudinal strain values. Previous studies showed that GLPS, decreased in AS, is even more affected in severe as compared to moderate AS [20–22]. Zito et al. [23], in a prospective study of 52 asymptomatic AS patients, showed that GLPS decrease is an independent predictor of the occurrence of the symptoms, need for AVR, or death. Moreover, the increase of longitudinal LV contractility expressed by GLPS is lower during exercise in patients with abnormal exercise test results [21]. After AVR followed by inverse left ventricle remodeling, the long-axis LV function and GLPS may improve [24,25].
In our study, GLPS (but not S′ velocity) was significantly lower in the symptomatic subgroup compared to asymptomatic patients. This finding is consistent with those obtained by Tongue et al. [26]. Despite left ventricle long-axis systolic function disturbances, LV diastolic dysfunction was also more pronounced in patients presenting clinical symptoms.
Limitations of the study
The control group consisted of patients referred to our Department who did not meet the inclusion criteria described above. This group was significantly younger than the study patients, because aortic stenosis occurs predominately in older subjects. This, however, does not influence our results, as it was shown that strain and strain rate do not change significantly with increasing age [27]. While ischemia obviously has an influence on LV function, the frequency of coronary artery disease in the study patients and in the control group was comparable. Moreover, there were no patients with regional wall motion abnormalities, thus LV function impairment in our groups were mainly caused by valvular disease, not by regional left ventricle ischemia.
Conclusions
In aortic stenosis patients, despite normal left ventricle ejection fraction, long-axis left ventricular function is impaired, which manifests in global longitudinal peak strain reduction. GLPS reveals that LV function impairment is more pronounced in symptomatic as compared to asymptomatic AS patients. The prognostic significance of early LV function impairment in aortic stenosis patients showed by GLPS should be determined in further studies.
Footnotes
Source of support: Departmental sources
References
- 1.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 2.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373:956–66. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Burwash IG, Legget ME, et al. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–70. doi: 10.1161/01.cir.95.9.2262. [DOI] [PubMed] [Google Scholar]
- 4.Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290–95. doi: 10.1161/CIRCULATIONAHA.104.495903. [DOI] [PubMed] [Google Scholar]
- 5.Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: implications for decision making. J Thorac Cardiovasc Surg. 2008;135:1270–79. doi: 10.1016/j.jtcvs.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 6.Leitman M, Lysyansky P, Sidenko S, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–29. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Dandel M, Lehmkuhl H, Knosalla C, et al. Strain and strain rate imaging by echocardiography – basic concepts and clinical applicability. Curr Cardiol Rev. 2009;5:133–48. doi: 10.2174/157340309788166642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korinek J, Wang J, Sengupta PP, et al. Two-dimensional strain-a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. J Am Soc Echocardiogr. 2005;18:1247–53. doi: 10.1016/j.echo.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease. The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–68. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- 12.Hill JA, Karimi M, Kutschke W, et al. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–69. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 13.Marcus ML, Doty DB, Hiratzka LF, et al. Decreased coronary reserve: a mechanism for angina pectoris in patients with aortic stenosis and normal coronary arteries. N Engl J Med. 1982;307:1362–66. doi: 10.1056/NEJM198211253072202. [DOI] [PubMed] [Google Scholar]
- 14.Heymans S, Schroen B, Vermeersch P, et al. Increased cardiac expression of tissue inhibitor of metalloproteinase-1 and tissue inhibitor of metalloproteinase-2 is related to cardiac fibrosis and dysfunction in the chronic pressure-overloaded human heart. Circulation. 2005;112:1136–44. doi: 10.1161/CIRCULATIONAHA.104.516963. [DOI] [PubMed] [Google Scholar]
- 15.Weidemann F, Herrmann S, Störk S, et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–84. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 16.Shimoni S, Gendelman G, Ayzenberg O, et al. Differential effects of coronary artery stenosis on myocardial function: the value of myocardial strain analysis for the detection of coronary artery disease. J Am Soc Echocardiogr. 2011;24:748–57. doi: 10.1016/j.echo.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Serri K, Reant P, Lafitte M, et al. Global and regional myocardial function quantification by two-dimensional strain: application in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2006;47:1175–81. doi: 10.1016/j.jacc.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 18.Mornoş C, Ruşinaru D, Manolis AJ, et al. The value of a new speckle tracking index including left ventricular global longitudinal strain and torsion in patients with dilated cardiomyopathy. Hellenic J Cardiol. 2011;52:299–306. [PubMed] [Google Scholar]
- 19.Marwick TH, Leano RL, Brown J, et al. Myocardial strain measurement with 2-dimensional speckle-tracking echocardiography: definition of normal range. JACC Cardiovasc Imaging. 2009;2:80–84. doi: 10.1016/j.jcmg.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki S, Daimon M, Miyazaki T, et al. Global longitudinal strain in relation to the severity of aortic stenosis: a two-dimensional speckle-tracking study. Echocardiography. 2011;28:703–8. doi: 10.1111/j.1540-8175.2011.01419.x. [DOI] [PubMed] [Google Scholar]
- 21.Donal E, Thebault C, O’Connor K, et al. Impact of aortic stenosis on longitudinal myocardial deformation during exercise. Eur J Echocardiogr. 2011;12:235–41. doi: 10.1093/ejechocard/jeq187. [DOI] [PubMed] [Google Scholar]
- 22.Lancellotti P, Donal E, Magne J, et al. Impact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: a two-dimensional speckle-tracking study. Eur J Echocardiogr. 2010;11:537–43. doi: 10.1093/ejechocard/jeq014. [DOI] [PubMed] [Google Scholar]
- 23.Zito C, Salvia J, Cusmà-Piccione M, et al. Prognostic significance of valvuloarterial impedance and left ventricular longitudinal function in asymptomatic severe aortic stenosis involving three-cuspid valves. Am J Cardiol. 2011;108:1463–69. doi: 10.1016/j.amjcard.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 24.Dinh W, Nickl W, Smettan J, et al. Reduced global longitudinal strain in association to increased left ventricular mass in patients with aortic valve stenosis and normal ejection fraction: a hybrid study combining echocardiography and magnetic resonance imaging. Cardiovasc Ultrasound. 2010;8:29. doi: 10.1186/1476-7120-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulsen SH, Søgaard P, Nielsen-Kudsk JE, Egeblad H. Recovery of left ventricular systolic longitudinal strain after valve replacement in aortic stenosis and relation to natriuretic peptides. J Am Soc Echocardiogr. 2007;20:877–84. doi: 10.1016/j.echo.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Tongue AG, Dumesnil JG, Laforest I, et al. Left ventricular longitudinal shortening in patients with aortic stenosis: relationship with symptomatic status. J Heart Valve Dis. 2003;12:142–49. [PubMed] [Google Scholar]
- 27.Ng AC, Tran da T, Newman M, et al. Left ventricular longitudinal and radial synchrony and their determinants in healthy subjects. J Am Soc Echocardiogr. 2008;21:1042–48. doi: 10.1016/j.echo.2008.05.002. [DOI] [PubMed] [Google Scholar]