Table 1.
Enantioselective Homocrotylation Scopea
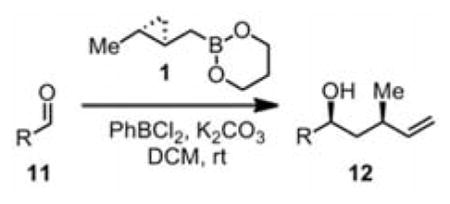
| ||||
|---|---|---|---|---|
| entry | aldehyde | time | % y | % eeb product/dr |
| 1 |
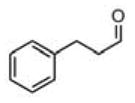 (11a) (11a) |
14 h | 83 | 97 |
| 2 | n-hept-CHO (11b) | 14 h | 89 | 97 |
| 3 | (C6H11)CHO (11c) | 14 h | 89 | 97 |
| 4 | i-PrCHO (11d) | 50 h | 72 (83) | 98 |
| 5 | t-BuCHO (11e) | 7 d | 62 (84) | 98 |
| 6 | PhCH2CHO (11f) | 14 h | 82 | 97 |
| 7 |
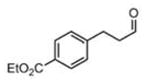 (11g) (11g) |
14 h | 89 | 97 |
| 8 |
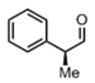 (11h) (11h) |
48 h | 83 |
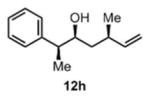 >20:1 drd |
| 9c |
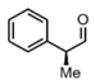 (11h)c (11h)c
|
48 h | 78 |
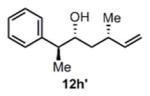 >20:1 drd |
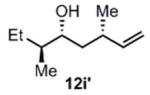
| 10 |
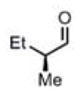 (11i) (11i) |
90 h | 76 (87) |
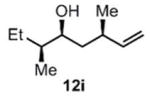 >99:1d |
| 11c |
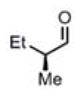 (11i)c (11i)c
|
90 h | 81 (96) |
 >99:1d |
3.0 equiv 1 and 1.5 equiv PhBCl2. Yields are isolated except where parentheses indicate NMR yields.
ee’s measured by chiral HPLC of alcohol or its benzoate derivative and are probably equal within error of measurement. Entries 1–7: no anti diastereomer was detected.
Ent-1 was used
Dr’s measured by RP HPLC or GC.
