Fig. 4.
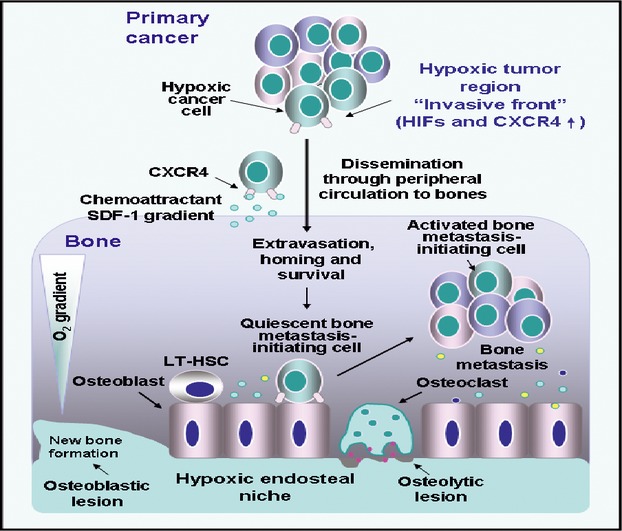
Proposed model of potential transforming events occurring in hypoxic cancer cells during epithelial cancer progression and bone metastasis. The up-regulated expression levels of stem cell-like phenotypes, HIFs, CXC chemokine receptor (CXCR4) and occurrence of the EMT programme in prostate or breast cancer cells within the hypoxic region at the invasive front of the primary tumour may lead to their invasion and dissemination through the peripheral circulation and homing at distant metastatic sites. More specifically, circulating prostate or breast cancer cells expressing high level of CXCR4 can preferentially disseminate and home to specific metastatic sites such as bones at least in part through the chemoattractant gradient formed by stromal cell-derived factor-1 (SDF-1) released by endothelial cells. The hypoxia-adapted prostate or breast cancer cells may compete with long-term haematopoietic stem cells (LT-HSCs) to occupy the hypoxic endosteal niche within BM and survive under a dormant state for a short or long period of time. The activation of dormant prostate or breast cancer cells may occur through the release of different growth factors and cytokines by cancer cells and stromal host cells under specific microenvironmental conditions. The activated prostate or breast cancer cells can give rise to the total tumour cell mass and skeletal metastasis formation. The bone metastases of prostate cancer cells are predominantly associated with the formation of osteoblastic lesions (bone formation), whereas bone metastases of breast cancer cells are generally related with the formation of osteolytic lesions (bone destruction).
