Figure 4.
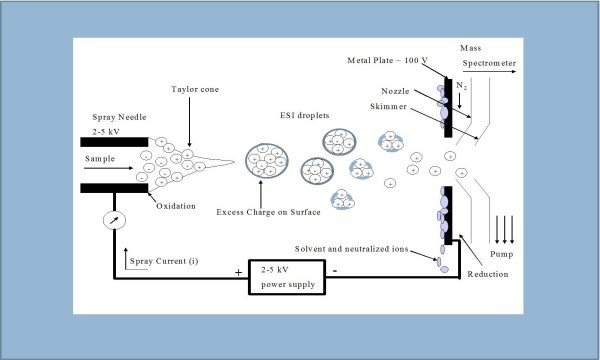
Illustration in a simple manner of the electrospray ionization process. The analyte solution is forced through the capillary, which has been supplied with high voltage. A Taylor cone is created due to the electric field between the capillary and the counter electrode, forming charged droplets of analyte ions and solvent. As these droplets travel towards the mass spectrometer the solvent evaporates creating analyte ions. When the solution that comprises the Taylor cone reaches the Rayleigh limit, at which point the Coulombic repulsion of the surface charge is equal to the surface tension of the solution, charged droplets are formed at the tip of the capillary. (Courtesy TE Tingholm Master Thesis 2005, PR Group, Odense Univ. Denmark).
