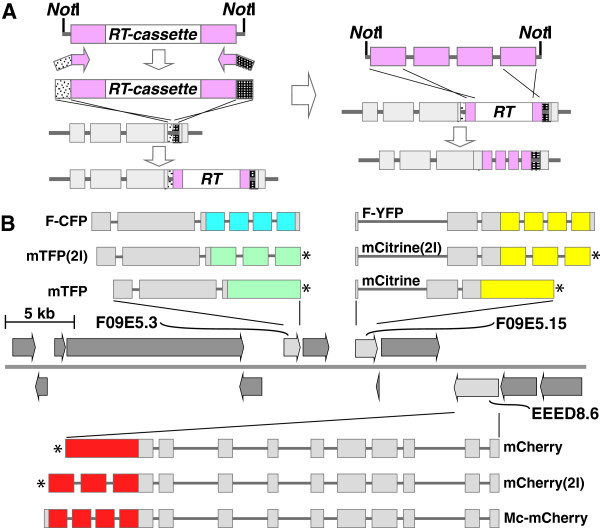
Figure 2.
Counter-selection recombineering-mediated generation of fosmid-based fluorescent protein-fusion reporter constructs. Panel A: schematic representation of the modified counter-selection protocol. A rpsL-tetA(C) (RT) counter-selection cassette, PCR-amplified from a NotI fragment excised from the appropriate pCC1Fos-based construct, flanked by approx. 200 bp from the 5′ and 3′ ends of the fluorescent protein reporter to be inserted (purple boxes) and terminal 50 bp homology arms (stippled boxes), is recombineered into the insertion site within the target gene via tetracycline selection of the positive marker (tetA(C)). In the subsequent replacement step a NotI fragment, containing the fluorescent protein coding sequence, replaces the inserted RT-cassette, via streptomycin selection conferred due to loss of the negative marker (rpsL), generating an in-frame gene::fp fusion. Panel B: a schematic representation, within the centre of the panel and drawn approximately to scale, illustrating sizes and orientations of the genes located on the insert of the fosmid clone WRM069dD11. The genes F09E5.3, F09E5.13 and EEED8.6 (light grey solid arrows) were tagged, respectively, with one of either F-CFP, mTFP1(2I) or mTFP1, F-YFP, mCitrine(2I) or mCitrine, or Mc-mCherry, mCherry(2I) or mCherry. Reporter gene fusions marked with an asterisk lack the 5 terminal codons of the target gene.