In patients with no chromosomal abnormalities (NC-AML) treated with chemotherapy, the detection of an FLT3-ITD and a mutation in NPM1 have helped in defining three risk categories, the worst corresponding to AML with an ITD and no NPM1 mutation.1-3 Recent studies on autologous hematopoietic stem cell transplantation (ASCT) have shown that it benefits patients with good and possibly intermediate risk prognostic factors. To define the best patient category for ASCT, we used the European Group for Blood and Marrow Transplantation (EBMT) registry to investigate the impact of FLT3/ITD presence and/or NPM1 gene mutation on the outcome of patients autografted in first complete remission (CR1).
We studied a total of 357 patients, 258 FLT3/ITD-negative and 99 FLT3/ITD-positive (63 with a normal karyotype) autografted in CR1 between January 2000 and December 2009. The information was restricted to the presence or absence of the molecular markers according to the techniques in use in each center. The registry contained no data on mutation levels.
Median follow up was 30 months (range 1-118). At diagnosis, patients with a detectable FLT3/ITD had a significantly higher white cell count of 53.7×109/L (range 0.6-265) vs. 12.4×109/L (range 0.5-300) (P<10-4). For 203 patients, information on both ITD and NPM1 mutation was available. Patients with a detectable FLT3/ITD were more likely to bear NPM1 mutation (59% versus 40%; P=0.03). Of these patients, 49% (n=100) were ITD-negative/NPM1-unmutated, 32.5% (n=66) were ITD-negative/NPM1-mutated, 11% (n=22) were ITD-positive/NPM1-mutated, and 7% (n=15) were ITD-positive/NPM1-unmutated.
The proportion of slow remitters (more than one induction chemotherapy course to achieve CR1) was higher in the group with an ITD (22% vs. 11%; P=0.04). The 3-year leukemia free survival (LFS) rate of the whole population was 47±3%. The relapse incidence (RI) rate was 47±3%, and non-relapse mortality (NRM) 6±1%. Results of the univariate analyses are shown in Table 1.
Table 1.
Main results of univariate analyses of prognostic factors at 3 years.
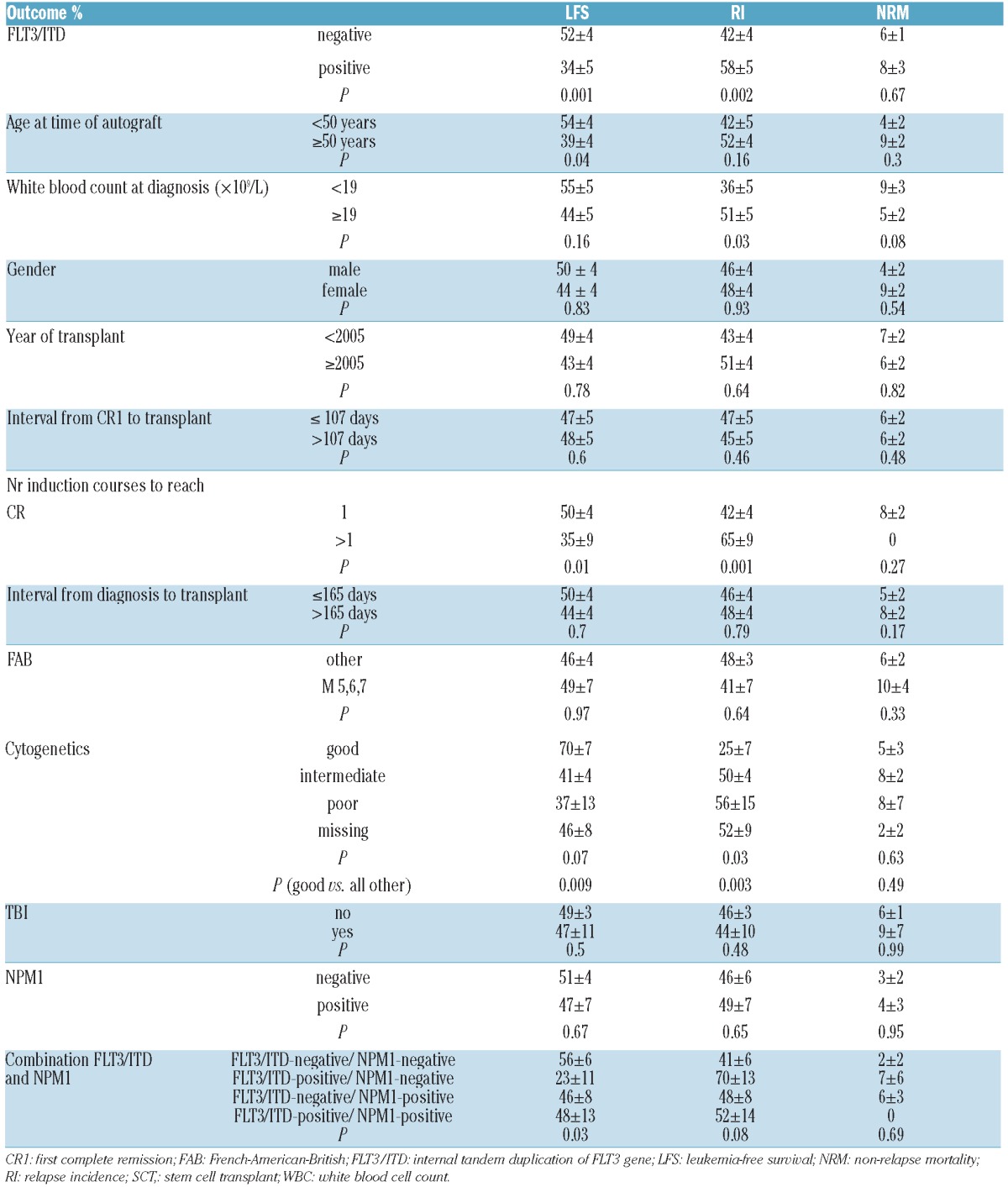
Patients without an FLT3/ITD had a lower RI (42±4% vs. 58±5%, P=0.002) and a better LFS (52±4% vs. 34±5%, P=0.001) and OS (66±3% vs. 47±6%, P=0.0006) than those with an ITD. Patients younger than 50 years of age had a better LFS (54±4% vs. 39±4%; P=0.04). Patients with good cytogenetics had the lowest RI (25±7%) and the best LFS (70±7%) compared to all other patients (RI 50±3%, P=0.003; LFS 43±3%, P=0.009). Rapid remitters had a lower RI (42±4% vs. 65±9%; P=0.001) and a better LFS (50±4% vs. 35±9%, P=0.01).
Figure 1 shows LFS for the four groups of patients defined by the two molecular markers. ITD-positive/NPM1-unmutated patients had the worst LFS (23±11%) and OS (37±18%), and the highest RI (70±13%) compared to the other three groups. Interestingly, in ITD-positive/NPM1-mutated patients, LFS was 48±13%, OS 69±13%, and RI 52±14%, no different from the outcome of ITD-negative/NPM1-unmutated patients.
Figure 1.
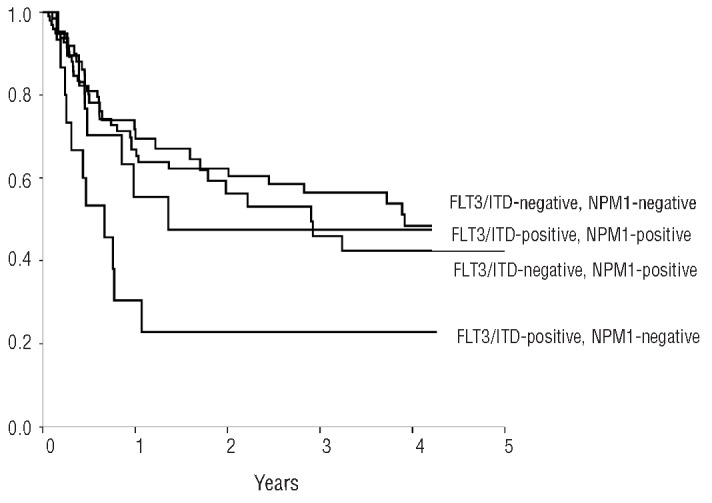
LFS according to FLT3/ITD and NPM1 status.
In multivariate analysis, both the presence of an FLT3/ITD and slow remitters were predictive of higher RI and lower LFS.
ASCT has been widely used for consolidation in patients with AML in complete remission, leading to long-term LFS for approximately 50% of patients when performed in CR1, and for approximately 30% of patients in CR2.4 Early randomized studies have shown ASCT to offer a lower RI than conventional chemotherapy but an inferior outcome than allogeneic transplantation from HLA identical siblings.5 Recently, interest has focused on the use of alternate donors and reduced intensity conditioning (RIC) with the possibility of considering allografting for patients up to 60-70 years of age, rather than considering ASCT as a first option.6 However, so far, there has been no indication that allogeneic hemopoietic stem cell transplantation using alternate donors and RIC results in outcomes superior to ASCT. Also, recent evaluations of the quality of life of allograft recipients have raised some concerns.7
Meanwhile, important prognostic factors for ASCT in AML in CR1 have been identified. Specifically, outcome has been shown to be better in younger patients, rapid remitters, good risk cytogenetics,8 patients autografted with bone marrow as compared to peripheral blood stem cells (PBSCs), and patients autografted with PBSCs who received sufficient in vivo purging.9 Recent identification of numerous molecular markers has made the detection and monitoring of MRD easier.
Approximately 30% of AML patients carry an ITD mutation. When treated with conventional chemotherapy, patients with this mutation and normal karyotype have reduced overall survival (OS). NPM1 mutations are found in approximately 30% of all cases and up to 50% of AML patients with normal karyotypes. The NPM1-mutated group has been shown to be associated with a higher CR rate, longer LFS, and longer OS.2,10
Although we found that patients with FLT3/ITD autografted in CR1 had a poorer outcome than those with wt FLT3 receptor, we also found that approximately 60% of patients with a concomitant NPM1 gene mutation still had an LFS of 48% and an OS of 69% at three years. This result may indicate that, in the context of autografting, the favorable impact of the NPM1 mutation may somehow compensate for the poor prognosis conveyed by the FLT3/ITD mutation. This finding is in agreement with other reports, albeit on much smaller scale.11 However, we have to acknowledge that the number of patients with information on both FLT3 ITD and NPM1 status was relatively small (22 patients with FLT3 ITD and mutant NPM1), and a meaningful multivariate analysis including the combined FLT3 ITD/NPM1 status as one variable was not possible. Recent studies have shown that NPM1 mutation quantification is a very reliable marker of MRD.12 Our study suggests ASCT to be a good post-remission therapeutic option for patients with an NPM1 gene mutation. This hypothesis remains to be tested in clinical trials.
Acknowledgments:
the authors thank the other major contributors to this study, Miguel Sanz (Valencia, Spain), Alberto Bosi (Florence, Italy), William Arcese (Roma, Italy), Giuseppe Milone (Catania, Italy), Emmanuelle Polge who coordinated the study, all data managers and the ALWP for collecting data from the EBMT Group centers.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Schlenk RF, Dohner K, Krauter J, Fröhling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909-18 [DOI] [PubMed] [Google Scholar]
- 2.Falini B, Martelli MP, Bolli N, Sportoletti P, Liso A, Tiacci E, et al. Acute myeloid leukemia with mutated nucleophosmin (NPM1): is it a distinct entity? Blood. 2011;117(4):1109-20 [DOI] [PubMed] [Google Scholar]
- 3.Schnittger S, Bacher U, Kern W, Alpermann T, Haferlach C, Haferlach T. Prognostic impact of FLT3-ITD load in NPM1 mutated acute myeloid leukemia. Leukemia. 2011;25(8):1297-304 [DOI] [PubMed] [Google Scholar]
- 4.Gorin NC. Autologous stem cell transplantation in acute myelocytic leukemia. Blood. 1998;92(4):1073-90 [PubMed] [Google Scholar]
- 5.Zittoun RA, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332(4):217-23 [DOI] [PubMed] [Google Scholar]
- 6.Hamadari M, Mohty M, Kharfan-Dabaja MA. Reduced intensity conditioning allogeneic hemopoietic cell transplantation in adults with acute myeloid leukemia. Cancer Control. 2011;18(4);237-45 [DOI] [PubMed] [Google Scholar]
- 7.Sun CL, Francisco L, Kawashima T, Leisenring W, Robison LL, Baker KS, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report form the bone marrow transplant survivor study. Blood. 2010;116(17):3129-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorin NC, Labopin M, Frassoni F, Milpied N, Attal M, Blaise D, et al. Identical outcome following autologous or allogeneic genoidentical hematopoietic stem cell transplantation in first remission of acute myelocytic leukemia carrying inversion 16 (inv 16) or t(8;21) A retrospective study from the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26(19):3183-8 [DOI] [PubMed] [Google Scholar]
- 9.Gorin NC, Labopin M, Reiffers J, Milpied N, Blaise D, Witz F, et al. Higher incidence of relapse in patients with acute myelocytic leukemia infused with higher doses of CD34+ cells from leukapheresis products autografted during the first remission. Blood. 2010;116(17):3157-62 [DOI] [PubMed] [Google Scholar]
- 10.Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M, et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML) Blood. 2006; 107(10):4011-20 [DOI] [PubMed] [Google Scholar]
- 11.Ferrara F, Izzo T, Criscuolo C, Riccardi C, Muccioli G, Viola A, et al. Favorable outcome in patients with acute myelogenous leukemia with the nucleophosmin gene mutation autografted after conditioning with high-dose continuous infusion of idarubicin and busulfan. Biol Blood Marrow Transplant. 2010;16(7):1018-24 [DOI] [PubMed] [Google Scholar]
- 12.Kronke J, Schlenk RF, Jensen KO, Tschürtz F, Corbacioglu A, Gaidzik VI, et al. Monitoring of Minimal Residual Disease in NPM1-Mutated Acute Myeloid Leukemia: A Study From the German-Austrian Acute Myeloid Leukemia Study Group. J Clin Oncol.2011;29(19):2709-16 [DOI] [PubMed] [Google Scholar]


