Abstract
Purpose
Suboptimal bowel preparation can result in decreased neoplasia detection, shortened surveillance intervals, and increased costs. We assessed bowel preparation recommendations and the relationship to self-reported proportion of suboptimal bowel preparations in practice; and evaluated the impact of suboptimal bowel preparation on colonoscopy surveillance practices. A random sample of a national organization of gastroenterologists in the U.S. was surveyed.
Methods
Demographic and practice characteristics, bowel preparation regimens, and proportion of suboptimal bowel preparations in practice were ascertained. Recommended follow-up colonoscopy intervals were evaluated for optimal and suboptimal bowel preparation and select clinical scenarios.
Results
We identified 6,777 physicians, of which 1,354 were randomly selected; 999 were eligible, and 288 completed the survey. Higher proportion of suboptimal bowel preparations/week (≥10 %) was associated with hospital/university practice, teaching hospital affiliation, >25 % Medicaid insured patients, recommendation of PEG alone and sulfate-free. Those reporting >25 % Medicare and privately insured patients, split dose recommendation, and use of MoviPrep® were associated with a <10 % suboptimal bowel preparations/week. Shorter surveillance intervals for three clinical scenarios were reported for suboptimal preparations and were shortest among participants in the Northeast who more often recommended early follow-up for normal findings and small adenomas. Those who recommended 4-l PEG alone more often advised <1 year surveillance interval for a large adenoma.
Conclusions
Our study demonstrates significantly shortened surveillance interval recommendations for suboptimal bowel preparation and that these interval recommendations vary regionally in the United States. Findings suggest an interrelationship between dietary restriction, purgative type, and practice and patient characteristics that warrant additional research.
Keywords: Colonoscopy, Purgatives, Suboptimal bowel preparation, Colonoscopy surveillance intervals, Survey
Introduction
Colorectal cancer is the third leading cause of cancer and cancer-related death in the United States [1]. Early detection and removal of precancerous polyps through screening has contributed to the steady decline in colorectal cancer incidence and mortality in recent years [1]. While there exist multiple modalities by which individuals can be screened for colorectal cancer, guidelines issued by multiple agencies consider colonoscopy as a preferred screening modality [2, 3]. As a result of increased colorectal cancer screening and detection of polyps, the demand for follow-up surveillance has also increased and now has become the most common reason for colonoscopy among adults aged 50 years and older in this country [4]. In fact, it is estimated that 25 % of all colonoscopies in the United States are for surveillance of polyps [5].
Bowel preparation is critical as diagnostic accuracy of the colonoscopy is dependent upon the ability to visualize the colon. However, reports indicate that, in as many as 19–30 % of colonoscopies [6–9], bowel preparation is suboptimal. Consequently, the endoscopist’s ability to reach the cecum is compromised, adenoma detection rate is lowered, the duration of the procedure is increased, and neoplasia may be missed [10].
Surveillance interval recommendations are dependent upon the number and pathology of the polyp(s) found which reflect the risk for future advanced adenomas (≥10 mm or villous features or ≥3 adenomas) and colorectal cancer [11, 12]. Guidelines indicate that follow-up of an index colonoscopy for findings within normal limits for an average-risk person is 10 years based on the rate at which advanced adenomas develop and the sensitivity of the colonoscopy [11]. Due to concern regarding inadequate inspection, suboptimal bowel preparation quality increases the duration of the colonoscopy and results in more repeat colonoscopies at shortened intervals which can drive up the cost of colonoscopy by as much as 12–22 % [9, 13, 14]. Shorter follow-up intervals also compromise system capacity to provide screening colonoscopy, and subject patients to further inconvenience and potentially expose them to greater risk of perforation and other adverse events [15–17].
In this study conducted among a nationally representative random cohort of American College of Gastroenterology (ACG) physicians, we sought to assess bowel preparation recommendations and the relationship to self-reported proportion of suboptimal bowel preparations in practice. Additionally, we examined the intervals at which these physicians recommend surveillance colonoscopy after suboptimal bowel preparation for six clinical scenarios and examined characteristics of those recommending shortened surveillance intervals for suboptimal bowel preparations. We hypothesized that factors related to the physician, the practice, the setting and the patient contribute to suboptimal bowel preparation and that interval recommendations will vary across the country. Only two studies to date have evaluated surveillance practices following suboptimal bowel preparation [13, 18] and no other studies have evaluated bowel preparation recommendations within the context of suboptimal bowel preparation quality.
Methods
This study was conducted between September 2010 and March 2011. With permission from the Research Committee of the American College of Gastroenterology in the United States, we obtained a complete U.S. membership list (n=10,228) consisting of names, credentials, and email and street addresses. Those members affiliated with pharmaceutical companies or non-medical entities, who had non-medical credentials (e.g., Ph.D.), or practiced pediatric gastroenterology were excluded (n=3,451). Of the remaining 6,777 members, we randomly selected a 20 % sample (n=1,355) using the random sample generator function of IBM SPSS version 19.
Three members (two previously responded in a pilot test of the study instrument and one duplicate entry) were removed post-selection of the random sample, resulting in 1352 physicians in our study sample. Of these, 26.2 % (n=354) were ineligible for the following reasons: unable to locate or had left the country (n=33), deceased (n=4), retired (n=27), and did not meet eligibility criteria (n=290, not a gastroenterologist, served a pediatric patient population, and did not perform screening colonoscopy routinely). Of 999 eligible participants, 288 (28.8 %) responded.
Each of the selected physicians was sent a personalized introductory letter stating an endorsement by the ACG to conduct this study, and a private link to an online survey over secure internet lines using Qualtrics™. A maximum of three emails were sent at approximately one month intervals, followed by two postal mailings approximately one month apart. Between email surveys and the postal surveys, each member was contacted by telephone to confirm the mailing address and eligibility. Completion of the survey was also encouraged during this call. A small incentive valued at $10 was offered to enhance participation.
The survey was developed and pilot-tested among gastroenterology fellows and faculty at our institution. Questions were grouped into four sections: demographic characteristics, practice characteristics, bowel preparation regimens used, and recommended follow-up colonoscopy interval based on clinical findings and bowel preparation quality. Proportion of suboptimal bowel preparations per week (none, 1–5 %, 6–10 %, 11–20 % and 21–30 %) was also assessed.
The recommended bowel preparation regimen for a healthy, average risk adult undergoing a screening colonoscopy was ascertained. Participants was asked questions related to the diet prescribed (clear liquid diet only vs. a more liberal diet consisting of clear liquids plus limited food types), use of split-dose bowel preparation, and purgative types recommended (4 l polyethylene glycol without additives [PEG, Colyte®, GoLytely®], sulfate-free PEG [NuLytely®, TriLyte], low volume PEG [Half-Lytely®], low volume PEG 3350 [MiraLAX®], and MoviPrep® [PEG with ascorbic acid]).
Additionally, we queried the participants about their personal recommendations for follow-up colonoscopy intervals based on a complete evaluation to the cecum. Six scenarios were presented that varied by the quality of the preparation and the clinical findings of the index screening on an average risk adult. In order to minimize interoperator variability, we defined a suboptimal bowel preparation as one that was “fair, poor, or inadequate” that may or may not have resulted in an aborted evaluation, whereas optimal bowel preparation was defined as an “adequate, good, or excellent bowel preparation that resulted in a complete evaluation to the cecum.” The clinical findings presented were “within normal limits,” “a single adenoma <10 mm,” and “a single adenoma ≥10 mm.” based on the U.S. Multi-Society Task Force on Colorectal Cancer and the American Cancer Society guidelines for colonoscopy surveillance after polypectomy [12]. Responses included “less than one year,” “1–2 years,” “3 years,” “4–5 years,” “10 years” and “other.”
Descriptive analyses were performed to examine demographic, practice characteristics, bowel preparation regimens, and recommended surveillance intervals. Self-reported suboptimal bowel preparations per week were dichotomized as low=<10 % vs. high=≥10 %. Bivariate analyses were conducted to examine which of the above factors were associated with self-reported proportion of suboptimal bowel preparation. The categorical data were analyzed with Pearson’s chi-square test using Yate’s continuity correction and for continuous variables, range, mean and standard deviation was calculated. We also evaluated associations between early follow-up recommendations for suboptimal preparations for each of three clinical scenarios. Significance was determined using p=0.05. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Participants ranged in age from 25 to 76 years with a mean age of 48.6 years (standard deviation [SD] 11.3) and the mean number of years of experience in performing colonoscopy was 17.2 (range 1–41 years, SD 10.8) (Table 1). The majority was white males who attended US medical school and were board certified in gastroenterology. More than 80 % of physicians reported that suboptimal bowel preparations occurred in 10 % or less of the colonoscopies they performed per week.
Table 1.
Characteristics of participating physicians (n=288)
| Total
|
||
|---|---|---|
| N | % | |
| Agea | ||
| 25–39 | 70 | 24.9 |
| 40–49 | 77 | 27.4 |
| 50–59 | 78 | 27.8 |
| 60+ | 56 | 19.9 |
| Gender | ||
| Male | 245 | 85.1 |
| Female | 43 | 14.9 |
| Race | ||
| White | 205 | 72.1 |
| Other | 80 | 27.9 |
| Medical school | ||
| U.S. | 209 | 78.6 |
| Non-U.S. | 57 | 21.4 |
| Specialty/Board Cert | ||
| G.I. | 242 | 85.8 |
| Non-G.I. | 40 | 14.2 |
| Years experience performing colonoscopyb | ||
| 0–6 | 67 | 24.5 |
| 7–16 | 71 | 25.9 |
| 17–25 | 72 | 26.3 |
| >26 | 64 | 23.4 |
| Proportion of suboptimal bowel preparations per week (%) | ||
| None | 6 | 2.2 |
| 1–5 | 125 | 46.8 |
| 6–10 | 86 | 32.2 |
| 11–20 | 38 | 14.2 |
| 21–30 | 12 | 4.5 |
Range 25–76 years; mean=48.6 years (SD 11.3)
Range 1–41 years; mean 17.2 years (SD 10.8)
The proportion of suboptimal bowel preparations (<10 % vs. ≥10 %) per week varied significantly by type of practice (p=0.0002) and affiliation with a teaching hospital affiliation (p=0.008) (Table 2). Of those in hospital/university practice types, 32.1 % reported ≥10 % suboptimal bowel preparations per week compared to 11.1 % in private practice, respectively. More than twice as many physicians affiliated with a teaching hospital reported the higher proportion of suboptimal bowel preparation per week (23.0 % vs. 10.1 %) compared with those with no such affiliation.
Table 2.
Comparison of practice characteristics and bowel preparation recommendations of participating physicians by self-reported level of suboptimal bowel preparations (n=266; <10 %, n=217 and ≥10 %, n=49)
| Total | % Suboptimal preparations1
|
χ2 | p value | |||||
|---|---|---|---|---|---|---|---|---|
| <10 % | ≥10 % | |||||||
|
|
|
|
||||||
| N | % | N | % | N | % | |||
| Practice characteristics | ||||||||
| Region (n=266) | 0.228 | 0.89 | ||||||
| Northeast | 86 | 32.3 | 69 | 80.2 | 17 | 19.8 | ||
| South | 94 | 35.3 | 78 | 83.0 | 16 | 17.0 | ||
| West and Hawaii | 86 | 32.3 | 70 | 81.4 | 16 | 18.6 | ||
| Setting (n=263) | 0.830 | 0.66 | ||||||
| Urban | 145 | 55.1 | 119 | 74.4 | 26 | 25.6 | ||
| Suburban | 100 | 38.0 | 80 | 80.0 | 20 | 20.0 | ||
| Rural | 18 | 6.8 | 16 | 88.9 | 2 | 11.1 | ||
| Type of practice (n=264) | 16.67 | 0.0002 | ||||||
| Private | 171 | 64.8 | 152 | 88.9 | 19 | 11.1 | ||
| Hospital/University | 81 | 30.7 | 55 | 67.9 | 26 | 32.1 | ||
| Other | 12 | 4.5 | 9 | 75.0 | 3 | 25.0 | ||
| Teaching hospital affiliation (n=264) | 6.953 | 0.008 | ||||||
| No | 99 | 37.5 | 89 | 77.0 | 10 | 10.1 | ||
| Yes | 165 | 62.5 | 127 | 89.9 | 38 | 23.0 | ||
| No. colonoscopies per week (n=264) | 2.868 | 0.41 | ||||||
| ≤10 | 27 | 10.2 | 21 | 77.8 | 6 | 22.2 | ||
| 11–20 | 87 | 33.0 | 68 | 78.2 | 19 | 21.8 | ||
| 21–30 | 93 | 35.2 | 81 | 87.1 | 12 | 12.9 | ||
| >30 | 57 | 21.6 | 46 | 80.1 | 11 | 19.9 | ||
| Patient insurance coverage (n=261) | ||||||||
| Medicaid | 9.541 | 0.002 | ||||||
| ≤25 % | 237 | 90.8 | 199 | 84.0 | 38 | 16.0 | ||
| >25 % | 24 | 9.2 | 14 | 58.3 | 10 | 41.7 | ||
| Medicare | 5.779 | 0.0016 | ||||||
| ≤25 % | 117 | 44.8 | 88 | 75.2 | 29 | 24.8 | ||
| >25 % | 144 | 55.2 | 125 | 86.8 | 19 | 13.2 | ||
| Private insurance | 4.552 | 0.033 | ||||||
| ≤25 % | 57 | 21.8 | 41 | 71.9 | 16 | 28.1 | ||
| >25 % | 204 | 78.2 | 172 | 84.3 | 32 | 15.7 | ||
| No insurance | 0.118 | 0.73 | ||||||
| ≤25 % | 257 | 96.3 | 210 | 81.7 | 47 | 18.3 | ||
| >25 % | 4 | 1.5 | 3 | 75.0 | 1 | 25.0 | ||
| Bowel preparation recommendations | ||||||||
| Dietary restrictionsb (n=265) | 0.952 | 0.33 | ||||||
| Liberal diet | 76 | 28.7 | 65 | 85.5 | 11 | 14.5 | ||
| Clear liquid only | 189 | 71.3 | 152 | 80.4 | 37 | 19.6 | ||
| Split dose (n=264) | 7.714 | 0.006 | ||||||
| No | 107 | 40.5 | 79 | 73.8 | 28 | 26.2 | ||
| Yes | 157 | 59.5 | 137 | 87.3 | 20 | 12.7 | ||
| Purgatives (n=264) | ||||||||
| 4 l PEG (no additives) | 4.254 | 0.04 | ||||||
| No | 115 | 43.6 | 101 | 87.7 | 14 | 12.2 | ||
| Yes | 149 | 56.4 | 115 | 77.2 | 34 | 22.8 | ||
| Sulfate-free PEG | 4.991 | 0.03 | ||||||
| No | 174 | 65.9 | 149 | 85.6 | 25 | 14.4 | ||
| Yes | 90 | 34.1 | 67 | 74.4 | 23 | 25.6 | ||
| Low volume PEG | 0.898 | 0.34 | ||||||
| No | 167 | 63.3 | 140 | 83.8 | 27 | 16.2 | ||
| Yes | 97 | 36.7 | 76 | 78.4 | 21 | 21.6 | ||
| Low volume PEG 3350 | 0.027 | 0.87 | ||||||
| No | 165 | 62.5 | 136 | 82.4 | 29 | 17.6 | ||
| Yes | 99 | 37.5 | 80 | 80.8 | 19 | 19.2 | ||
| MoviPrep® | 3.745 | 0.05 | ||||||
| No | 157 | 59.5 | 122 | 77.7 | 35 | 22.3 | ||
| Yes | 107 | 40.5 | 94 | 87.9 | 13 | 12.1 | ||
PEG=4 l polyethylene glycol without additives, Colyte®, GoLYTELY®; sulfate-free PEG = NuLYTELY®, TriLyte®; low volume PEG = HalfLytely®; low volume PEG 3350 = MiraLAX®; MoviPrep® = PEG with ascorbic acid
Self-reported proportion of suboptimal (fair, poor, or inadequate) bowel preparations per week
Clear liquid only diet vs. liberal diet = clear liquid diet plus certain allowable food types
In evaluating the proportion of the patient population by insurance coverage (>25 % vs. ≤25 %), the majority of physicians reported having relatively few Medicaid-insured (9.2 %) and uninsured patients (1.5 %). Physicians with greater numbers of Medicaid insured individuals more often reported a higher proportion of suboptimal bowel preparation (41.7 % vs. 16.0 %, p=0.002). The reverse was observed among physicians reporting higher proportions of patients insured by Medicare (13.2 % vs. 24.8 %, p=0.0016) and private insurance (15.7 % vs. 28.1 %, p=0.033).
Nearly three-quarters of all participants (71.3 %) recommended only a clear liquid diet prior to colonoscopy and 59.5 % recommended split dosing of the purgative (Table 2). Polyethylene glycol (4 l PEG without additives, Colyte®, or GoLYTELY®) was the most commonly recommended purgative (56.4 %), followed by MoviPrep® (PEG with ascorbic acid) (40.5 %). Not recommending split dosing of the purgative was associated with a higher proportion of suboptimal bowel preparations per week (26.2 % vs. 12.7 %, p=0.006). With regard to purgatives, recommendation of 4 l of PEG without additives and sulfate-free PEG was associated with the higher proportion of suboptimal bowel preparations compared to not using these purgatives (4 l PEG without additives, 22.8 % vs. 12.2 %, p=0.04, and sulfate-free PEG 25.6 % vs. 14 %, p=0.03). Comparing those who did not recommend MoviPrep® (12.1 %) to those who did (22.3 %), higher proportion of suboptimal bowel preparation was associated with not recommending MoviPrep® (p=0.05).
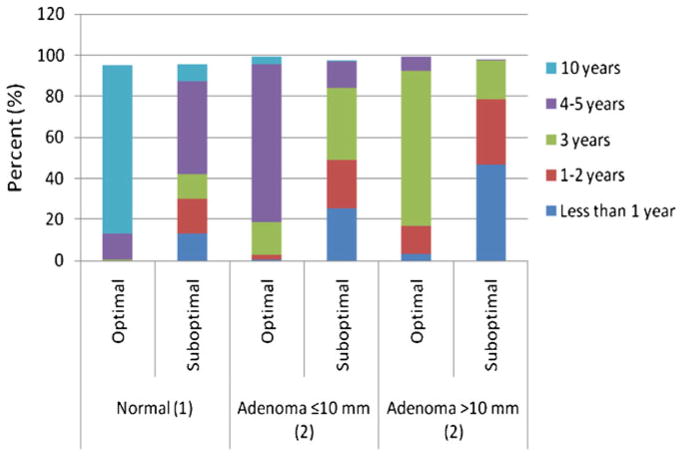
For an average-risk adult with an optimal bowel preparation and findings within normal limits, 82.4 % recommended follow-up in 10 years; for a single adenoma <10 mm, 77.2 % recommended follow-up in 4–5 years; and for a single adenoma ≥10 mm, 76.0 % recommended follow-up in 3 years (Fig. 1). For the same average risk adult with a suboptimal preparation and findings within normal limits, 42.1 % recommended follow-up in <4–5 years; for a single adenoma <10 mm, 84.2 % recommended follow-up <3 years; and for a single adenoma ≥10 mm, 46.5 % recommended follow-up <1 year. Differences between interval recommendations at each level of bowel preparation quality (<10 % vs. ≥10 %) were statistically significant for findings within normal limits (p<0.0007), for a small adenoma <10 mm (p<0.0001), and for a large adenoma ≥10 mm (p<0.0001). Those reporting a higher proportion of suboptimal preparations more often recommended shorter intervals for each clinical scenario.
Fig. 1.
Self-reported recommended colonoscopy surveillance intervals for findings (within normal limits, a single adenoma ≤10 mm, and a single adenoma >10 mm) after index colonoscopy with suboptimal preparation on an average risk individual
Shortened intervals were defined as <4–5 years for findings within normal limits, <3 years for a single small adenoma (≤10 mm) and <1 year for a single large adenoma (>10 mm) (Table 3). In the setting of a suboptimal preparation, participants in the Northeast were significantly more likely to recommend follow-up in less than 4–5 years for normal findings (53.6 % vs. 39.4 %, p=0.04) and less than 3 years for a single small adenoma ≤10 mm (59.5 % vs. 45.6 %, p=0.048). Those who recommended 4 l of PEG without additives were more aggressive overall in the their surveillance for each of the three clinical scenarios and this was statistically significant for large adenomas with 67.2 % reporting <1 year surveillance interval for a large adenoma (p=0.008)
Table 3.
Comparison of various physician and practice characteristics and purgative recommendations with self-reported shortened surveillance intervals for suboptimal preparation quality and clinical findings
| Within normal limits <4–5 years
|
Single adenoma ≤10 mm <3 years
|
Single adenoma >10 mm <1 year
|
||||
|---|---|---|---|---|---|---|
| N (%) | p value | N (%) | p value | N (%) | p value | |
| Region | 0.04 | 0.048 | 0.11 | |||
| Northeast | 45 (53.6) | 50 (59.5) | 47 (55.3) | |||
| Other | 69 (39.4) | 82 (45.6) | 79 (43.9) | |||
| Practice type | 0.53 | 0.56 | 0.07 | |||
| Private | 70 (42.4) | 80 (48.5) | 71 (42.8) | |||
| Other | 44 (47.3) | 52 (53.1) | 54 (55.1) | |||
| Teaching hospital affiliation | 0.15 | 0.92 | 0.30 | |||
| Yes | 77 (48.1) | 84 (50.9) | 83 (50.3) | |||
| No | 37 (38.1) | 48 (49.5) | 42 (42.9) | |||
| Proportion of suboptimal preparations per weeka | 0.63 | 0.80 | 0.89 | |||
| ≤10 | 21 (48.8) | 22 (47.8) | 21 (45.7) | |||
| >10 | 90 (43.5) | 107 (51.2) | 101 (48.1) | |||
| Diet | ||||||
| Clear liquid | 108 (97.3) | 0.16 | 127 (98.5) | 0.99 | 121 (98.2) | 1.00 |
| Low residue | 29 (25.7) | 0.89 | 28 (21.4) | 0.27 | 25 (20.0) | 0.10 |
| Split dose | 0.73 | 0.12 | 0.79 | |||
| Yes | 64 (43.0) | 70 (45.8) | 102 (47.7) | |||
| No | 50 (45.9) | 62 (56.1) | 18 (43.9) | |||
| Purgative | ||||||
| 4 l PEG no additives | 69 (61.1) | 0.35 | 81 (61.8) | 0.28 | 84 (67.2) | 0.008 |
| Sulfate-free PEG | 37 (32.7) | 1.00 | 42 (32.1) | 0.83 | 47 (37.6) | 0.16 |
| Low volume PEG | 42 (37.2) | 0.84 | 45 (34.4) | 0.64 | 47 (37.6) | 0.70 |
| Low volume PEG 3350 | 41 (36.3) | 0.98 | 47 (35.9) | 0.94 | 46 (36.8) | 1.00 |
| MoviPrep® | 42 (37.2) | 0.22 | 53 (40.5) | 0.84 | 51 (40.8) | 0.98 |
Suboptimal preparation defined as inadequate, fair or poor preparation
PEG=4 l polyethylene glycol without additives, Colyte®, GoLYTELY®; sulfate-free PEG = NuLYTELY®, TriLyte; low volume PEG = Halflytely®; low volume PEG 3350 = Miralax®; MoviPrep® = PEG with ascorbic acid
Self-reported proportion of suboptimal (fair, poor, or inadequate) bowel preparations per week
Discussion
We found that, in this survey of a nationally representative sample of U.S. gastroenterologists, the surveillance intervals recommended for suboptimal bowel preparation scenarios deviated significantly from recommendations for optimal preparation and those of published surveillance guidelines [11, 12, 19]. Furthermore, the intervals were shortest among physicians in the Northeast section of the country for findings within normal limits and for a single, low-risk adenoma, neither of which can be empirically justified [11, 12]. Those practicing in hospital/university settings or who were affiliated with a teaching hospital reported higher proportions of suboptimal preparations. This finding may be reflective of more diverse patient populations, types of insurance coverage accepted, use of particular preparation types, and procedure volume in the hospital/university setting vs. private practice.
Moreover, we documented that dietary restrictions and purgative type and dosing also impact the proportion of suboptimal bowel preparations reported. Several studies have documented the lack of adherence to guideline follow-up recommendations [13, 18, 20, 21] demonstrating a trend toward shortened intervals that is perhaps instigated by malpractice concerns, lack of knowledge [22, 23], or personal preference over guideline recommendations [24]. None inquired about bowel preparation quality with respect to interval recommendations, however. Our study is the first to survey a sample of U.S. gastroenterologists regarding surveillance intervals for suboptimal bowel preparation and to evaluate diet and purgative recommendations with regard to self-reported proportion of suboptimal bowel preparation in practice.
To date, only two other studies have evaluated surveillance intervals for suboptimal bowel preparation quality. The first presented a series of colon segment images with cleansing quality varying from impeccable to intermediate quality and asked participants to recommend surveillance intervals for normal findings and two small polyps on index colonoscopy. In this study of 78 physicians attending a conference in Israel, there was considerable inter-observer variation with respect to the interpretation of the bowel quality depicted in the images. Rather than repeating the procedure for the suboptimally prepared colon, most recommended a shorter interval for follow-up with a shift to the left of 2–3 years (p<0.001 for trend) [13]. The second study was a retrospective chart review of 126 medical practices in North Carolina to determine the post-polypectomy surveillance recommendations [18]. For patients with small adenomas, more than one-third of patients were told to return sooner than the recommended guidelines. Bowel preparation quality was missing in 32 % of the records reviewed; nonetheless, the authors found that surveillance interval for low-risk, small adenomas was shorter for those with less than excellent preparation compared to excellent preparation [18].
Our findings suggest that factors, such as the physician’s practice type, affiliation with a teaching hospital, dietary restrictions, type of purgative recommended, and patient type as determined by insurance status, may play an important role in the proportion of suboptimal bowel preparations. Others have found that not following bowel preparation instructions, the timing of the start of the procedure, inpatient vs. outpatient status, type of insurance coverage, constipation, use of tricyclic antidepressants, male gender, marital status, and comorbidities such as a history of cirrhosis, stroke, or dementia are all independent predictors of suboptimal bowel preparation [9, 25–27]. We propose that it is feasible that factors related to the physician and his/her practice and patient characteristics, considered in combination rather than individually, influence the proportion of suboptimal bowel preparations encountered in practice.
It is interesting that the self-reported proportion of suboptimal bowel preparations in practice among this cohort of gastroenterologists was far lower than that reported in the literature [28]. This underreporting of the prevalence of suboptimal bowel preparations may be due in part to how suboptimal bowel preparation was defined in this survey (“fair, poor or inadequate”). Alternatively, this underestimation may be reflective of a reporting bias, with respondents hesitant to reveal actual proportions or may be related to a general lack of awareness of the occurrence of suboptimally prepared bowels in their own practices.
Strengths of this study include the use of a national group of gastroenterologists selected randomly from the membership list of a prestigious professional organization. Furthermore, we examined demographic and practice information as well as explored dietary and purgative recommendations in relation to suboptimal bowel preparation prevalence and surveillance interval recommendations. Despite apparent underreporting of the proportion of suboptimal bowel preparations in practice, we observed associations between region and purgative and shortened surveillance intervals recommended for patients with suboptimal preparations.
There were, of course, limitations to this study as well. The response rate to our survey was low at 28.8 % but, is commensurate with the findings of several others [29–34] (response rates of 32.7 %, 27.1 %, undetermined, 10 %, 5.8 %, and 11 %, respectively). These studies represent a wide range of topics using varying methods to obtain information on participants’ opinions, knowledge, and behavior. But, for whatever reason, whether continual bombardment with requests for survey participation, lack of interest/time, or lack of proper incentives, response rate among this group has been demonstrated to be low. The opinions of the members of this organization, however, are held in high esteem and appear to reflect accurately upon current issues in the field. Additionally, our sample size was small and, thus, we may have had insufficient power to detect an effect for other covariates. Our conclusions are based on self-reported behavior of this cohort of physicians regarding the specific scenarios presented in the survey and not upon objective evidence of actual practice that is likely guided by risk assessments, patient symptomology, and previous pathology. That teaching hospital affiliation was associated with proportion of suboptimal bowel preparations, even as underreported as it was in this study, may be difficult to interpret as this term likely encompasses a broad spectrum of practitioner types and patient populations. We also found that the majority of physicians in our study reported ≤10 % of colonoscopies performed weekly had suboptimal bowel preparation, inconsistent with the literature in this area. Nevertheless, we did find evidence suggestive of a relationship between self-reported high proportion of suboptimal bowel preparation and the type of physician practice, dietary restrictions, and purgative recommendations.
When low-risk findings are encountered during a colonoscopy with suboptimal bowel preparation, gastroenterologists in our study were more likely, particularly in the Northeast, to shorten interval recommendations aggressively. Ramifications of shortened surveillance intervals are numerous—for example, they may compromise the capacity of the system to perform screening colonoscopy [22] and expose patients to potential procedural harm [14]. Additionally, there exists no evidence to support greater detection of pre-cancerous adenomas or cancer to justify these shorter intervals [12] particularly as interval cancers are associated with endoscopists’ adenoma detection rate and not with rate of cecal intubation [35]. Rather than shortening the surveillance interval, others have suggested that suboptimal bowel preparations should be followed by a repeat the preparation and procedure immediately [27, 36].
Our study demonstrates the significantly shortened surveillance interval recommendations in the case of suboptimal bowel preparation. Our findings suggest an interrelationship between dietary restriction and purgative type preferences and recommendations, and gastroenterology practice characteristics that warrants additional research. Further investigation into the development of best practice guidelines regarding surveillance of low-risk findings discovered upon colonoscopy with suboptimal bowel preparation is needed to ensure efficient and cost-effective utilization of this procedure.
Acknowledgments
This work was supported by a National Cancer Institute at the National Institutes of Health fellowship (R25 CA094601) to C.H. Basch; a National Center for Research Resources (NCRR) at the National Institutes of Health grant (KL2 RR024157) to B. Lebwohl; American Cancer Society (RSGT-09-012-01-CPPB) grant to C.E. Basch; and National Cancer Institute at the National Institutes of Health (K07 151769) grant to F. Kastrinos.
Footnotes
Conflict of interests None.
Contributor Information
Grace Clarke Hillyer, Email: gah28@columbia.edu, Department of Epidemiology, Mailman School of Public Health, Columbia University, 722 W. 168th Street, New York, NY 10032, USA.
Corey H. Basch, Department of Public Health, William Paterson University, Wayne, NJ, USA
Benjamin Lebwohl, Division of Digestive and Liver Diseases, Columbia University, New York, NY, USA. Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Charles E. Basch, Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA. Department of Health & Behavior Studies, Teachers College, Columbia University, New York, NY, USA
Fay Kastrinos, Division of Digestive and Liver Diseases, Columbia University, New York, NY, USA. Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Beverly J. Insel, Department of Epidemiology, Mailman School of Public Health, Columbia University, 722 W. 168th Street, New York, NY 10032, USA
Alfred I. Neugut, Department of Epidemiology, Mailman School of Public Health, Columbia University, 722 W. 168th Street, New York, NY 10032, USA. Herbert Irving Comprehensive Cancer Center, College of Physicians and Surgeons, Columbia University, New York, NY, USA. Division of Hematology and Oncology of the Department of Medicine, Columbia University, New York, NY, USA
References
- 1.American Cancer Society. Colorectal cancer facts and figures, 2011–2013. American Cancer Society; Atlanta: 2011. [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 3.Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. Am J Gastroenterol. 2000;95:868–877. doi: 10.1111/j.1572-0241.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62:875–883. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman DA, De Garmo PL, Fleischer DE, Eisen GM, Helfand M. Patterns of endoscopy use in the United States. Gastroenterol. 2000;118:619–624. doi: 10.1016/s0016-5085(00)70269-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen LA, Santos S, Jandorf L, Christie J, Castillo A, Winkel G, et al. A program to enhance completion of screening colonoscopy among urban minorities. Clin Gastroenterol Hepatol. 2008;6:443–450. doi: 10.1016/j.cgh.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–79. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 8.Kazarian ES, Carreira FS, Toribara NW, Denberg TD. Colonoscopy completion in a large safety net health care system. Clin Gastroenterol Hepatol. 2008;6:438–442. doi: 10.1016/j.cgh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55:2014–2020. doi: 10.1007/s10620-009-1079-7. [DOI] [PubMed] [Google Scholar]
- 10.Froehlich F, Wietlisbach V, Gonvers J-J, Burnand B, Vader J-P. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 11.Winawer SJ, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterol. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 12.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Horin S, Bar-Meir S, Avidan B. The impact of colon cleanliness assessment on endoscopists’ recommendations for follow-up colonoscopy. Am J Gastroenterol. 2007;102:2680–2685. doi: 10.1111/j.1572-0241.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- 14.Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696–1700. doi: 10.1111/j.1572-0241.2002.05827.x. [DOI] [PubMed] [Google Scholar]
- 15.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169–174. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 16.Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 18.Ransohoff DF, Yankaskas B, Gizlice Z, Gangarosa L. Recommendations for post-polypectomy surveillance in community practice. Dig Dis Sci. 2011;56:2623–2630. doi: 10.1007/s10620-011-1791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D, et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130:1865–1871. doi: 10.1053/j.gastro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264–271. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 21.Mulder SA, Ouwendijk RJ, van Leerdam ME, Nagengast FM, Kuipers EJ. A nationwide survey evaluating adherence to guidelines for follow-up after polypectomy or treatment for colorectal cancer. J Clin Gastroenterol. 2008;42:487–492. doi: 10.1097/MCG.0b013e31809e703c. [DOI] [PubMed] [Google Scholar]
- 22.Boolchand V, Olds G, Singh J, Singh P, Chak A, Cooper GS. Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med. 2006;145:654–659. doi: 10.7326/0003-4819-145-9-200611070-00007. [DOI] [PubMed] [Google Scholar]
- 23.Saini SD, Nayak RS, Kuhn L, Schoenfeld P. Why don’t gastroenterologists follow colon polyp surveillance guidelines?: results of a national survey. J Clin Gastroenterol. 2009;43:554–558. doi: 10.1097/MCG.0b013e31818242ad. [DOI] [PubMed] [Google Scholar]
- 24.Krist AH, Jones RM, Woolf SH, Woessner SE, Merenstein D, Kerns JW, et al. Timing of repeat colonoscopy: disparity between guidelines and endoscopists’ recommendation. Am J Prev Med. 2007;33:471–478. doi: 10.1016/j.amepre.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 25.Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797–1802. doi: 10.1111/j.1572-0241.2001.03874.x. [DOI] [PubMed] [Google Scholar]
- 26.Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25:373–384. doi: 10.1111/j.1365-2036.2006.03212.x. [DOI] [PubMed] [Google Scholar]
- 27.Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from American Society of Colon and Rectal Surgeons, American Society for Gastrointestinal Endoscopy, and Society of American Gastrointestinal and Endoscopic Surgeons. Gastrointest Endosc. 2006;63:894–909. doi: 10.1016/j.gie.2006.03.918. [DOI] [PubMed] [Google Scholar]
- 28.Ko CW, Riffle S, Shapiro JA, Saunders MD, Lee SD, Tung BY, et al. Incidence of minor complications and time lost from normal activities after screening or surveillance colonoscopy. Gastrointest Endosc. 2007;65:648–656. doi: 10.1016/j.gie.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Cattau EL., Jr Colonoscopy capacity in Tennessee: potential response to an increased demand for colorectal cancer screening. Tenn Med. 2010;103(37–38):40. [PubMed] [Google Scholar]
- 30.Cohen LB, Wecsler JS, Gaetano JN, Benson AA, Miller KM, Durkalski V, et al. Endoscopic sedation in the United States: results from a nationwide survey. Am J Gastroenterol. 2006;101:967–974. doi: 10.1111/j.1572-0241.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- 31.Sorbi D, Gostout CJ, Peura D, Johnson D, Lanza F, Foutch PG, et al. An assessment of the management of acute bleeding varices: a multicenter prospective member-based study. Am J Gastroenterol. 2003;98:2424–2434. doi: 10.1111/j.1572-0241.2003.t01-1-07705.x. [DOI] [PubMed] [Google Scholar]
- 32.Spergel JM, Book WM, Mays E, Song L, Shah SS, Talley NJ, et al. Variation in prevalence, diagnostic criteria, and initial management options for eosinophilic gastrointestinal diseases in the United States. J Pediatr Gastroenterol Nutr. 2011;52:300–306. doi: 10.1097/MPG.0b013e3181eb5a9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trindade AJ, Morisky DE, Ehrlich AC, Tinsley A, Ullman TA. Current practice and perception of screening for medication adherence in inflammatory bowel disease. J Clin Gastroenterol. 2011;45:878–882. doi: 10.1097/MCG.0b013e3182192207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasan SK, Coukos JA, Farraye FA. Vaccinating the inflammatory bowel disease patient: deficiencies in gastroenterologists knowledge. Inflamm Bowel Dis. 2011;7:2536–2540. doi: 10.1002/ibd.21667. [DOI] [PubMed] [Google Scholar]
- 35.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 36.Bond JH. Should the quality of preparation impact postcolonscopy follow-up recommendations? Am J Gastroenterol. 2007;102:2686–2687. doi: 10.1111/j.1572-0241.2007.01483.x. [DOI] [PubMed] [Google Scholar]