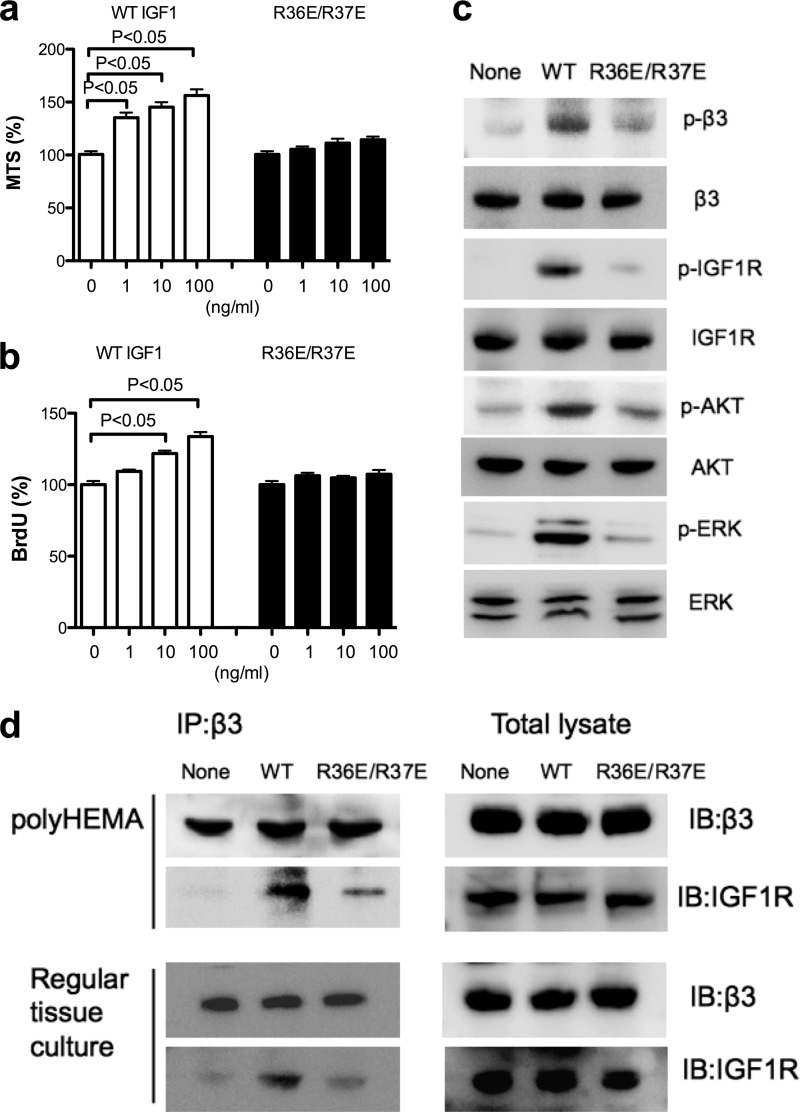
FIGURE 3.
The effect of R36E/R37E on IGF1 signaling in β3-CHO cells in anchorage-independent conditions. a, cell survival. β3-CHO cells were plated in polyHEMA-coated plates, serum-starved in DMEM, and cultured with WT IGF or R36E/R37E (1, 10, and 100 ng/ml) for 48 h. Cell survival was measured by MTS assays. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. b, cell proliferation. β3-CHO cells were plated in polyHEMA-coated plates, serum-starved in DMEM, and cultured with WT IGF or R36E/R37E (1, 10, and 100 ng/ml) for 24 h. BrdU incorporation was measured. The data are shown as mean ± S.E. (n = 6). Statistical analysis was performed using ANOVA and Tukey's multiple comparison test. c, intracellular signals induced by IGF1. p, phospho. β3-CHO cells were plated in polyHEMA-coated plates and serum-starved in DMEM for 3 h. Cells were stimulated with WT IGF1or R36E/37E (100 ng/ml) for 30 min. Cell lysates were analyzed by Western blotting. d, coprecipitation of IGF1R with integrin β3. β3-CHO cells were plated in polyHEMA-coated plates and serum-starved in DMEM for 3 h or in regular tissue culture plates and serum-starved in DMEM for 12 h. Cells were stimulated with WT IGF1or R36E/37E (100 ng/ml) for 30 min. β3 was immunopurified (IP) with anti-β3 antibodies from cell lysates. The immunoprecipitated materials or total cell lysates were analyzed with anti-IGF1R or β3 antibodies by Western blotting (IB).