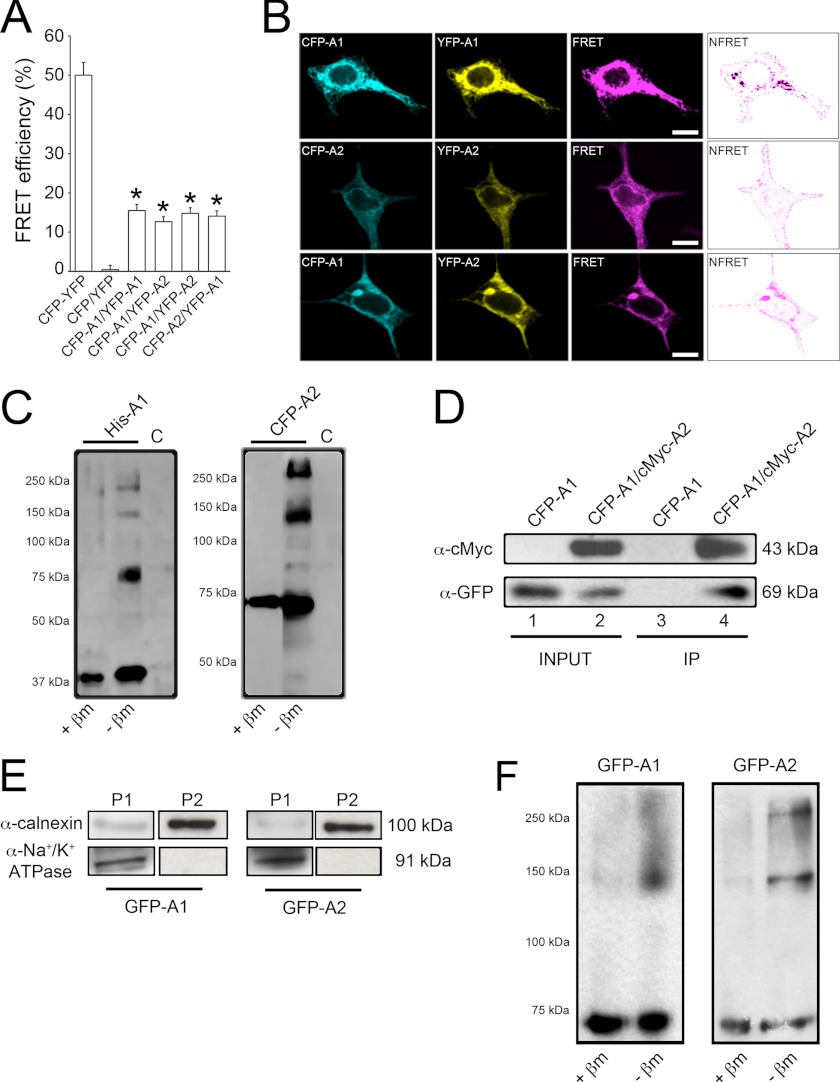
FIGURE 3.
Evaluation of AdipoR1 and AdipoR2 interaction. A, shown are measurements of FRET efficiency in fixed HEK293AD cells transfected with different ECFP and Venus-YFP constructs. FRET efficiency values were calculated as described under “Experimental Procedures.” Cells expressing ECFP and Venus-YFP coupled in-frame within the same plasmid construct were used as the positive control (46 cells). Cells expressing ECFP and Venus-YFP empty vectors were used as negative control (44 cells). FRET was measured in cells co-transfected with ECFP-AdipoR1/Venus-YFP-AdipoR1 (71 cells), ECFP-AdipoR2/Venus-YFP-AdipoR2 (67 cells), ECFP-AdipoR1/Venus-YFP-AdipoR2 (63 cells), or ECFP-AdipoR2/Venus-YFP-AdipoR1 (62 cells) under basal culture conditions. Only cells displaying Venus-YFP/ECFP ratios close to or equal to 1 were included in the analysis. Results presented are the average ± S.E. of the number of cells indicated (*, p < 0.001 versus negative control). B, shown is a PixFRET map of FRET efficiencies yielded by AdipoR homo- and heteromers. Normalized FRET (NFRET) channels (rightmost panels) reveal that protein interaction occurs mainly at the plasma membrane, although some positive signal is also present in the ER. Scale bars, 5 μm. C, protein extracts from HEK293AD cells were transfected with His6-tagged AdipoR1 (left panel) or ECFP-tagged AdipoR2 (right panel) and electrophoresed in the presence or absence of the reducing agent β-mercaptoethanol. As the control (C), cells were transfected with a construct coding for the His6 tag alone. D, immunoprecipitation (IP) of the AdipoR1/AdipoR2 complex is shown. Immunoprecipitation was carried out with lysates prepared from HEK293AD cells co-expressing the ECFP-AdipoR1/cMyc-AdipoR2 combination. For control purposes, HEK293AD cells were transfected with ECFP-AdipoR1 alone. After cell lysis, protein extracts were immunoprecipitated with monoclonal anti-cMyc antibody and then immunoblotted using anti-cMyc or anti-GFP polyclonal antibodies. Monoclonal anti-cMyc antibody co-precipitated ECFP-AdipoR2 in ECFP-AdipoR1/cMyc-AdipoR2 co-expressing cells (lane 4) but not in cells expressing ECFP-AdipoR1 alone (lane 3). E, assessment of ER membrane-enriched protein extracts by subcellular fractionation is shown. Protein extracts from GFP-AdipoR1- or GFP-AdipoR2-transfected cells were centrifuged at 600 × g for 10 min, 15,000 × g for 5 min, and 100,000 × g for 60 min. Pellets from the second (P1) and third (P2) centrifugation steps were immunostained against the ER membrane marker calnexin (top panels) and the plasma membrane marker Na+/K+-ATPase (bottom panels). F, subcellular fractionation of HEK293AD cell extracts was performed to further investigate the presence of AdipoR complexes in ER membrane-enriched fractions. ER-enriched fractions were incubated in the presence or absence of β-mercaptoethanol (βm), electrophoresed, and immunostained against GFP to identify AdipoR monomers and multimers.