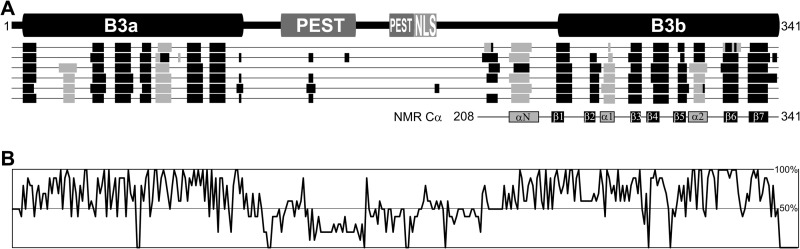
FIGURE 1.
Schematic of VRN1. A, VRN1 consists of two B3 domains of ∼100 residues each. Between them are two putative PEST-rich protein turnover domains and a Lys-rich nuclear localization sequence (NLS). Below VRN1 (from top to bottom) are the outputs from secondary structure prediction programs jalign, jfreq, jhmm, jnet, jpssm, and jpred, which suggest that only the B3 domains and a region preceding B3b are structured. The VRN1(208–341) protein has been studied in solution by NMR, and the Cα shift data (6) correlated well with these predictions. The secondary structural elements are numbered for later reference. B, summary of the percentage conservation within an alignment of VRN1 and its 10 most similar proteins from the GenBankTM Data Bank. Conservation is high at the B3 domains but also in the region preceding B3b. For the full sequence alignment this panel was based on, see supplemental Fig. S1.