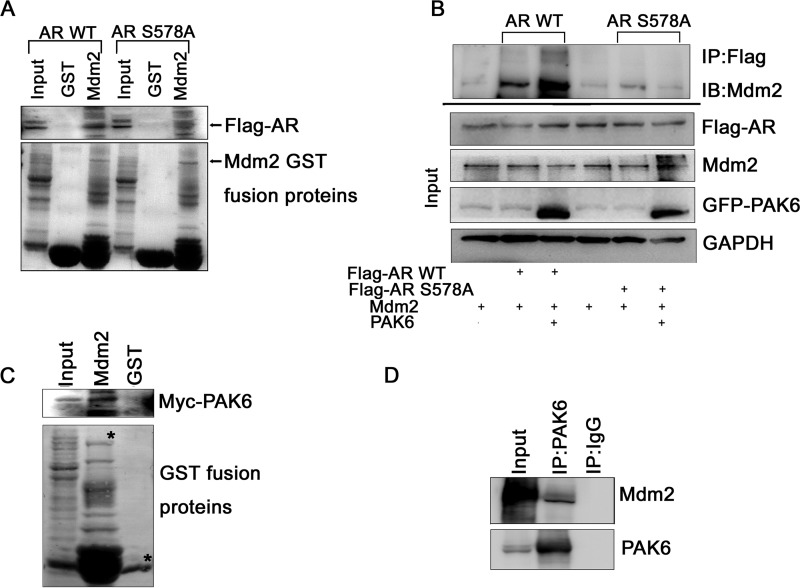
FIGURE 4.
PAK6-mediated phosphorylation of AR enhances AR association with Mdm2. A, Mdm2 interacted strongly with AR WT but much more weakly with AR S578A mutant. In vitro translated pcDNA-FLAG-AR WT/S578A (505–919 amino acids) was incubated with GST-Mdm2 as indicated and analyzed with anti-FLAG antibody. GST served as control. B, Mdm2 associated stronger with AR WT than AR S578A mutant in the presence of PAK6. COS7 cells were co-transfected with pcDNA-FLAG-AR WT/S578A (505–919 amino acids) in combination with pcDNA-EGFP-PAK6 and Mdm2 as indicated, treated with 10 nm DHT. 30 μg of total lysates were set aside as input; equal amounts of lysates of protein were immunoprecipitated (IP) with anti-FLAG antibody and immunoblotted (IB) with anti-Mdm2 antibody. C, GST pulldown assay showed that PAK6 and Mdm2 interact with each other in vitro. In vitro translated pcDNA3.1-Myc-His-PAK6 was incubated with GST-Mdm2 and analyzed with anti-Myc antibody. GST served as control. D, immunoprecipitation assay confirmed that PAK6 associates with Mdm2 in CWR22Rv1 cells. Equal amounts of CWR22Rv1 cell lysates were immunoprecipitated with anti-PAK6 or IgG. Endogenous PAK6 and Mdm2 were immunoblotted with anti-PAK6 and anti-Mdm2 antibodies.