FIGURE 1.
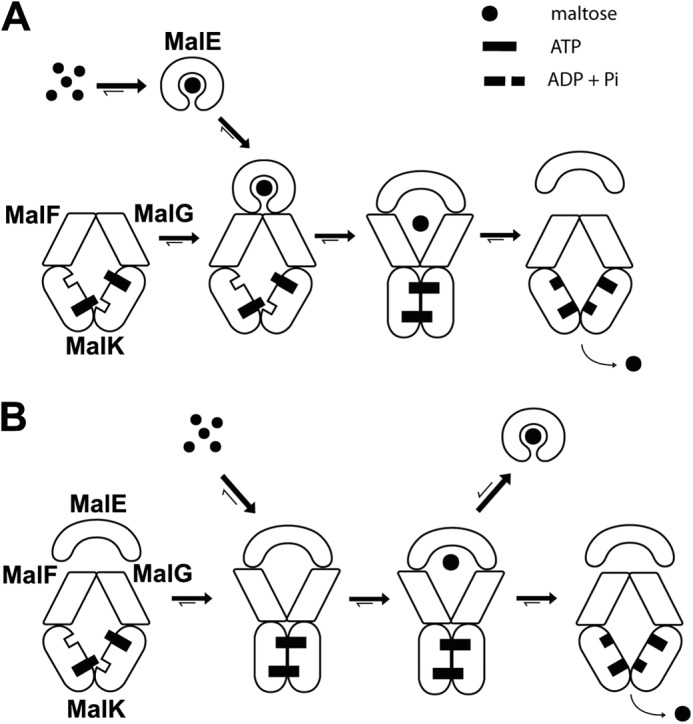
Two opposed models for maltose transport. A, in the conventional model, closed liganded MalE triggers the outward facing conformation. MalE binds maltose (Kd = ∼2 μm) and then associates with the inward facing transporter (Kd > ∼45 μm). The transition to the outward facing conformation facilitates the opening of MalE and the release of maltose to the MalFG cavity. Upon ATP hydrolysis, the transporter returns to the inward facing state and maltose is released in the cytosol. In this model, MalE facilitates the pairing of MalK and the ATP hydrolysis step. B, in the proposed new model, ATP alone triggers the outward facing conformation. The outward facing transporter binds unliganded MalE with a high affinity (Kd = ∼50–80 nm). Maltose then binds to the MalE-MalFGK2 assembly (Kd = ∼120 μm). Upon ATP hydrolysis, the transporter returns to the inward facing conformation, and maltose is released in the cytosol. In this model, MalE stimulates the return of the transporter to the inward facing conformation. If ATP hydrolysis does not take place immediately, or if maltose is present in excess, MalE acquires its closed liganded conformation and dissociates from the transporter (negative autoregulation).
