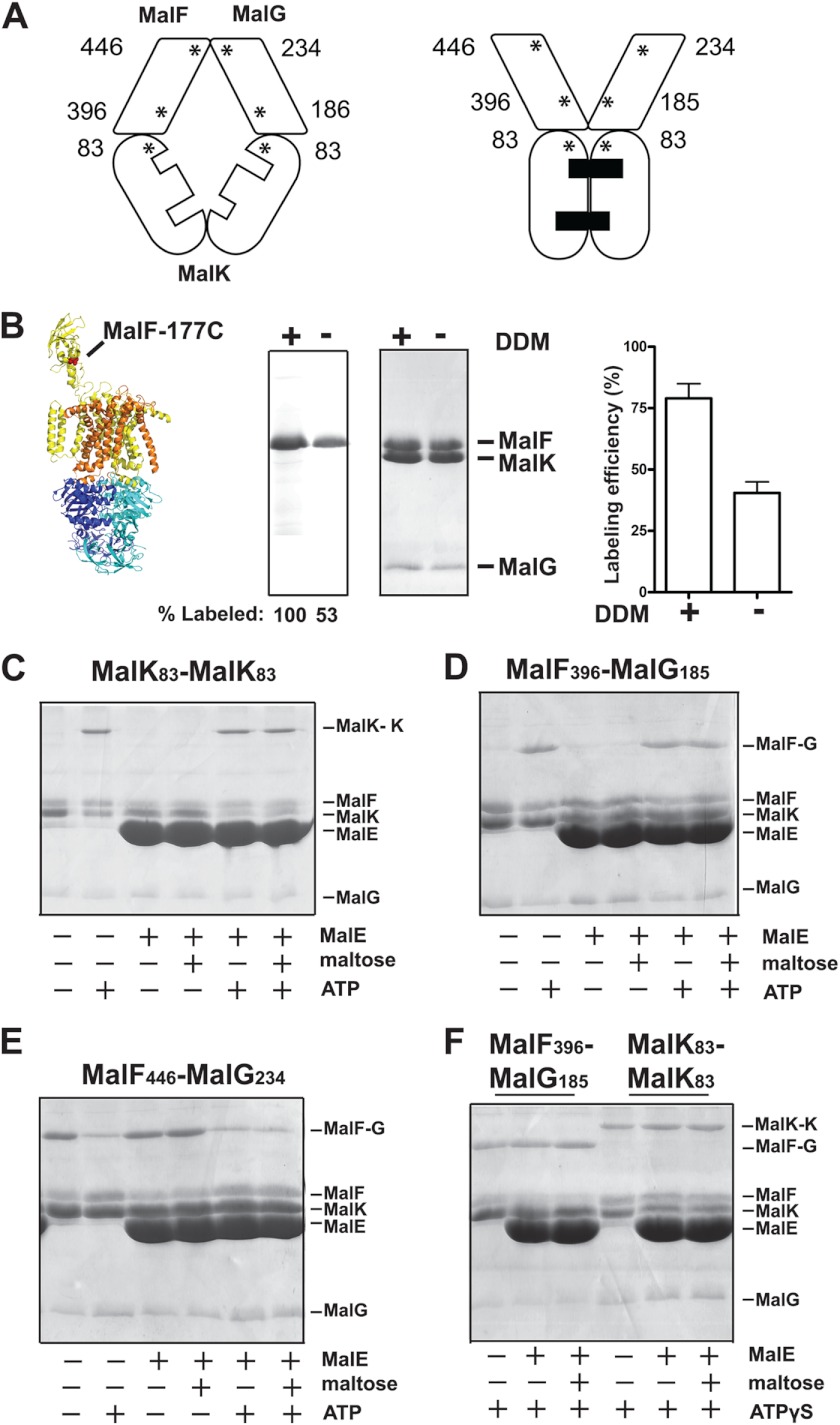
FIGURE 2.
ATP triggers the transporter outward facing conformation. A, the asterisks indicate the location of the cysteine residues introduced in MalF, MalG, and MalK and their relative positions in the inward and outward facing conformations. B, orientation of the MalFGK2 complex in proteoliposomes. The labeling efficiency of residue MalFT177C (in mutant background MalKC40S) was assayed using the membrane-impermeable reagent 5-IAF in the presence or absence of DDM. The samples were analyzed by 15% SDS-PAGE followed by fluorescence scanning (left panel) or Coomassie Blue staining (right panel). The fluorescence of MalFT177C when labeled in the presence of DDM was normalized to 100%. The labeling efficiency of MalFT177C was determined by absorbance spectroscopy (498 nm), using an extinction co-efficient of 75, 500 cm−1 m−1. C–E, the indicated transporters reconstituted in proteoliposomes were incubated with the homobifunctional cross-linker BMOE (50 μm) in the indicated conditions. The samples were treated with N-ethylmaleimide (5 mm) prior to analysis by 15% SDS-PAGE and Coomassie Blue staining. F, cross-linking results for the pairs MalF396/MalG185 and MalF446/MalG234 in the presence of ATPγS.