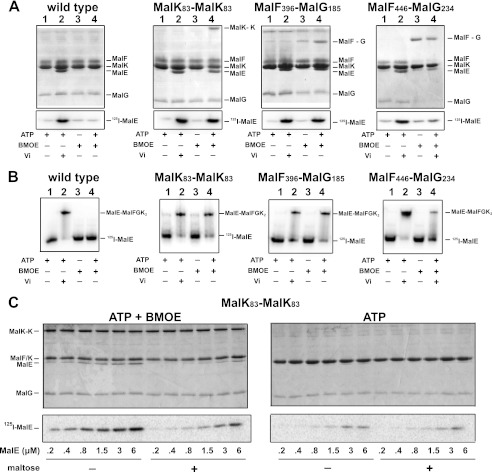
FIGURE 3.
ATP controls the binding of MalE to the transporter. A, the binding of MalE to MalFGK2 in proteoliposomes was analyzed by co-sedimentation assay. [125I]MalE (∼10,000 cpm, 10 μm) was incubated with MalFGK2 proteoliposomes (2 μm) in the presence of ATP, vanadate, and BMOE as indicated (room temperature, 10 min). The samples were diluted 25-fold in 20 mm Tris-HCl, pH 8.0, before ultracentrifugation (100,000 × g, 1 h). The fraction of MalE bound to MalFGK2 was analyzed by 15% SDS-PAGE followed by Coomassie Blue staining (top panels) or autoradiography (bottom panels). B, the binding of [125I]MalE to MalFGK2 in nanodiscs was analyzed by native gel electrophoresis. [125I]MalE (∼5,000 cpm, 0.5 μm) was incubated with MalFGK2 nanodiscs (1 μm) in the presence of ATP, vanadate, and BMOE as indicated (room temperature, 10 min). The samples were analyzed by native gel electrophoresis and autoradiography. C, maltose promotes the dissociation of MalE when MalFGK2 is stabilized in the outward facing state. The binding of MalE to MalFGK2 in proteoliposomes was analyzed by sedimentation assay. The indicated amount of [125I]MalE was incubated with MalFGK2 (pair MalK83-MalK83) in proteoliposomes in the presence of ATP or ATP and BMOE (room temperature, 10 min). The samples were diluted 25-fold in 20 mm Tris-HCl, pH 8, with or without 1 mm maltose. The fraction of MalE bound to MalFGK2 was isolated by ultracentrifugation (100,000 × g, 1 h). The amount of bound MalE was analyzed by 12% SDS-PAGE followed by Coomassie Blue staining (top panels) or autoradiography (bottom panels). Note that MalF and MalK are migrating at the same position on 12% SDS-PAGE.