FIGURE 8.
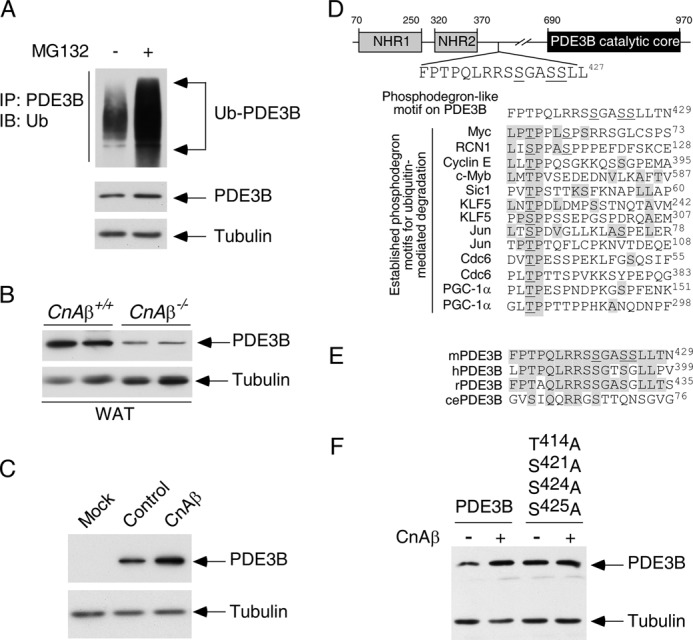
Calcineurin phosphatase regulates expression of phosphodiesterase 3B. A, COS cells were transiently transfected with PDE3B and ubiquitin. Ubiquitin conjugation of PDE3B was determined by co-immunoprecipitation assays. The presence of ubiquitin (Ub) in PDE3B immunoprecipitates (IP) was analyzed by immunoblot (IB) analysis. The effect of proteosome inhibitor MG132 (10 μm) was also shown. B, extracts prepared from CnAβ−/− and CnAβ+/+ white adipose tissues (WAT) were subjected to immunoblotting analysis to determine endogenous expression of PDE3B. The expression levels of tubulin were used as controls. C, PDE3B was co-expressed with CnAβ in COS cells. The expression levels of PDE3B were determined by immunoblot analysis. The expression levels of tubulin were used as controls. D, schematic illustration of the location of the FPTPQLRRSSGASSLLT phosphodegron on PDE3B. A potential phosphorylation site (Thr414) on PDE3B is shown. Adjacent Ser421, Ser424, and Ser425 of PDE3B, which were found phosphorylated previously (55), are also indicated. Sequences of known phosphodegrons on other proteins are also illustrated. Conserved amino acid residues and previously identified phosphorylated Thr/Ser are shaded and underlined, respectively. E, sequence alignment of the potential phosphodegron FPTPQLRRSSGASSLLT from mouse, human, rat, and C. elegans is shown. F, wild type or Ala414,421,424,425 PDE3B was co-expressed with CnAβ in COS cells. The expression levels of PDE3B were determined by immunoblot analysis. The expression levels of tubulin were used as controls.
