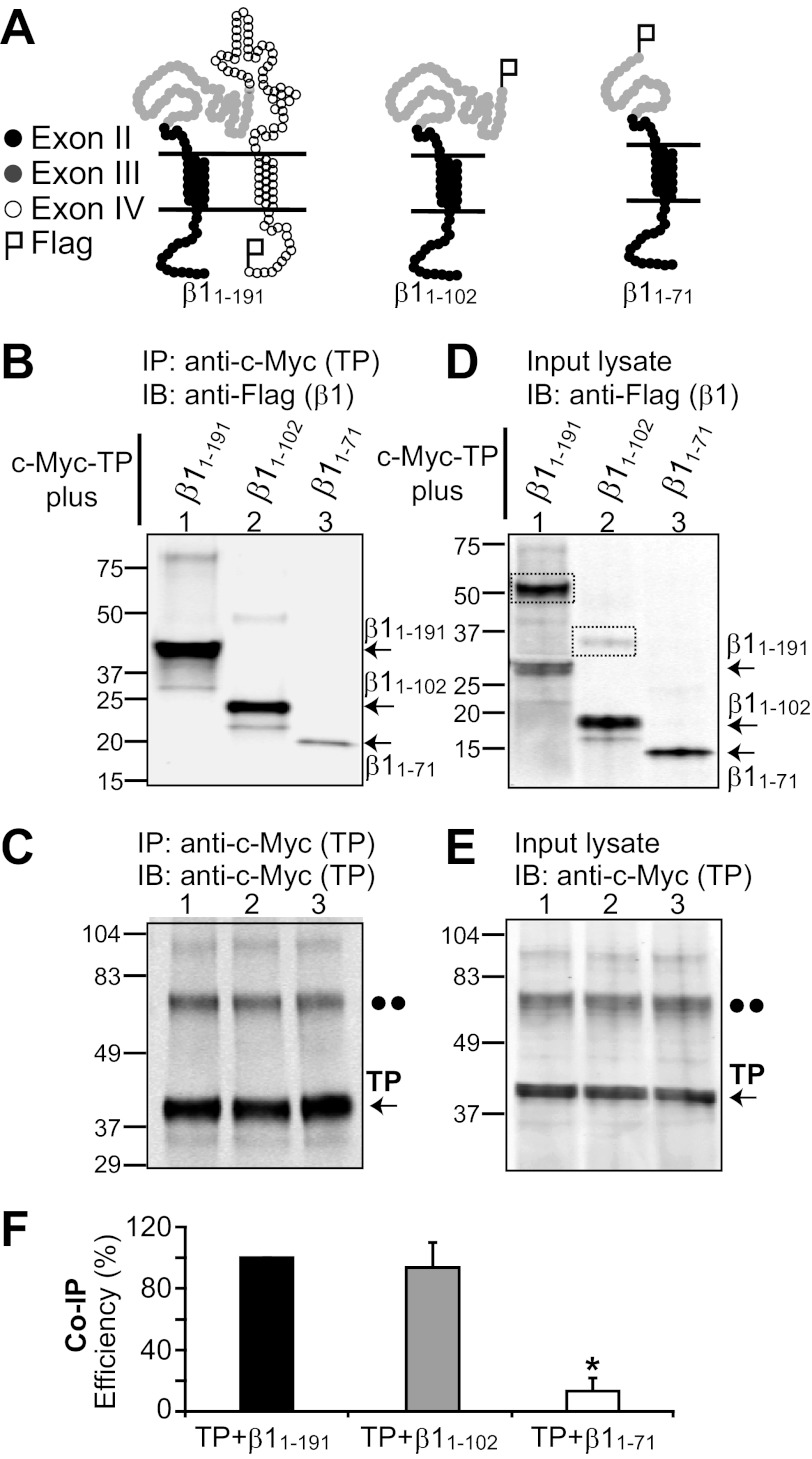
FIGURE 3.
β1 domain involved in β1-TP interaction. A, topological schemes of wild-type β1 (β1(1–191)) and deletion constructs β1(1–102) and β1(1–71). Black circles represent amino acids 1–45 encoded by exon II, gray circles represent amino acids 46–102 encoded by exon III, and open circles represent amino acids 103–191 encoded by exon IV. β1 constructs were tagged with a FLAG epitope at the C terminus. B–F, HEK293T cells were transfected with c-Myc-TP and β1 constructs (4 μg of each plasmid/2 ml of medium in a 60-mm dish) for co-immunoprecipitation analysis. B, TP effectively pulls down β1(1–191) (wild-type) and the β1(1–102) deletion construct. β1(1–102) from mouse or human yielded similar results. In contrast, TP associated poorly with β1(1–71). TP immunoprecipitation (IP) was performed with anti-c-Myc polyclonal antibody, and immunoblotting (IB) was carried out with anti-FLAG monoclonal antibody. C, the control shows similar TP immunoprecipitation in all instances, confirming the poor association of TP with β1(1–71). Anti-c-Myc polyclonal antibody was used for immunoprecipitation, and anti-c-Myc monoclonal antibody was used for immunoblotting. D and E, double-labeled immunoblot of the corresponding input lysates (18 μg of protein/lane) showing the expression levels of β1 constructs and TP, respectively. The dashed boxes mark β1-subunit dimers. The double dots mark the position corresponding to the putative TP dimer. F, mean values of co-immunoprecipitation (Co-IP) efficiency (n = 3) calculated as follows: Co-IP efficiency (%) = 100 × (density of co-immunoprecipitated β1 construct)/((density of immunoprecipitated TP) × (density of β1 construct in input cell lysate)). *, significantly different from TP + β1(1–191) (p < 0.01).