Fig. 5. Endogenously produced Δ7/11 is glycosylated.
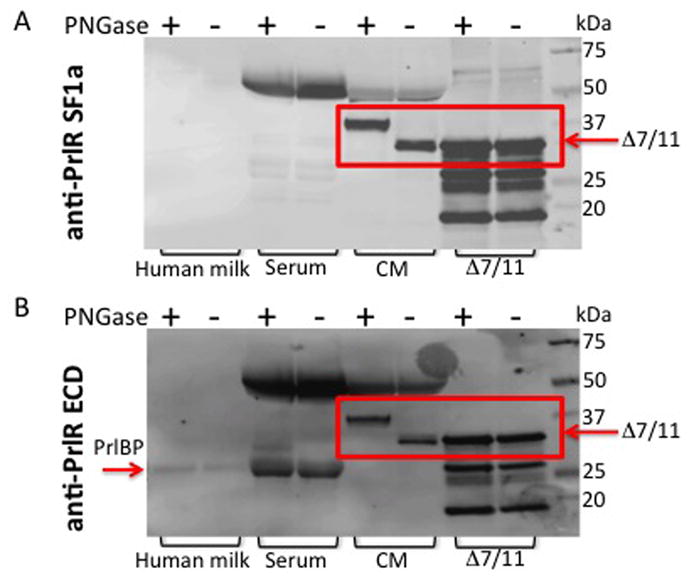
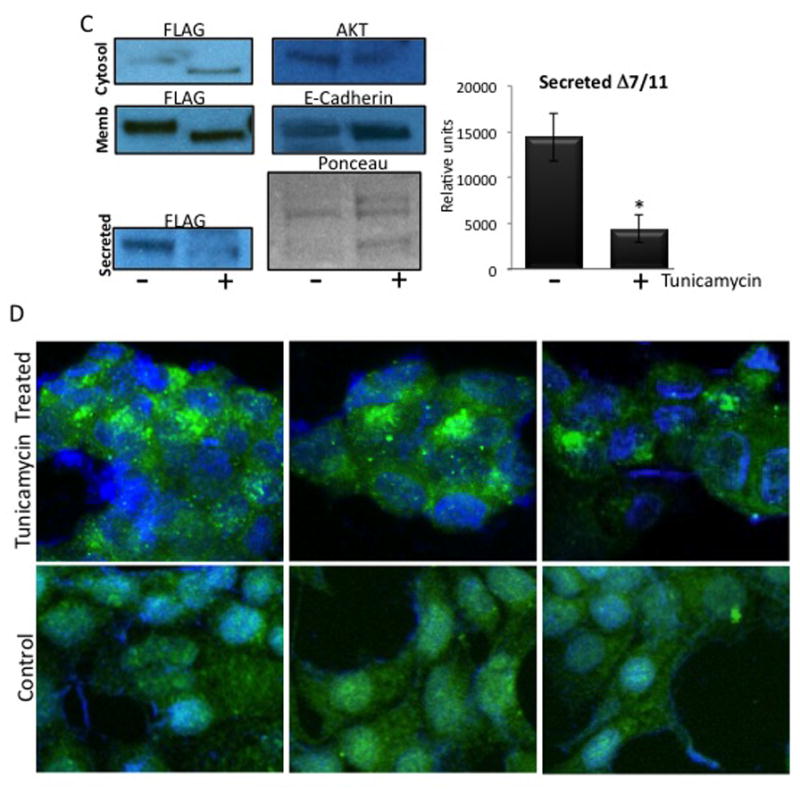
(A) Samples were de-glycosylated with PNGase F treatment, and then subjected to western blot analysis using an antibody directed against the prolactin receptor short form 1a (SF1a), which recognizes Δ7/11. (B) Membranes were stripped and re-probed using an antibody that recognizes the extracellular domain common to the prolactin receptor isoforms (anti-PrlR ECD). Serum = human serum. CM = concentrated conditioned media from CHO cells stably expressing Δ7/11. Δ7/11 = recombinant Δ7/11 produced in E. coli. Approximate molecular weights: hPRLBP, 25 kDa; glycosylated Δ7/11, 39.1 kDa; glycosylated Δ7/11, 40.6 kDa. (C) T47D cells transiently transfected with FLAG-tagged Δ7/11 were treated +/− tunicamycin (500 ng/ml) for 48 hrs. Conditioned media were collected and concentrated, cell lysates were separated into membrane and cytosolic fractions. Representative western blots of cytosolic, membrane, and secreted proteins are shown. AKT, E-Cadherin and Ponceau S stained membrane are shown as loading controls for cytosolic, membrane, and conditioned media, respectively. Histogram shows the quantitation of the amount of Δ7/11 secreted from cells from three independent experiments (*P<0.05). (D) Representative images of T47D cells transiently transfected with FLAG-tagged Δ7/11, treated +/− tunicamycin for 48 hrs, and then analyzed via immunocytofluorescence. FLAG-tagged Δ7/11 stained with Alexa488 (green); DNA stained with DAPI (blue).
