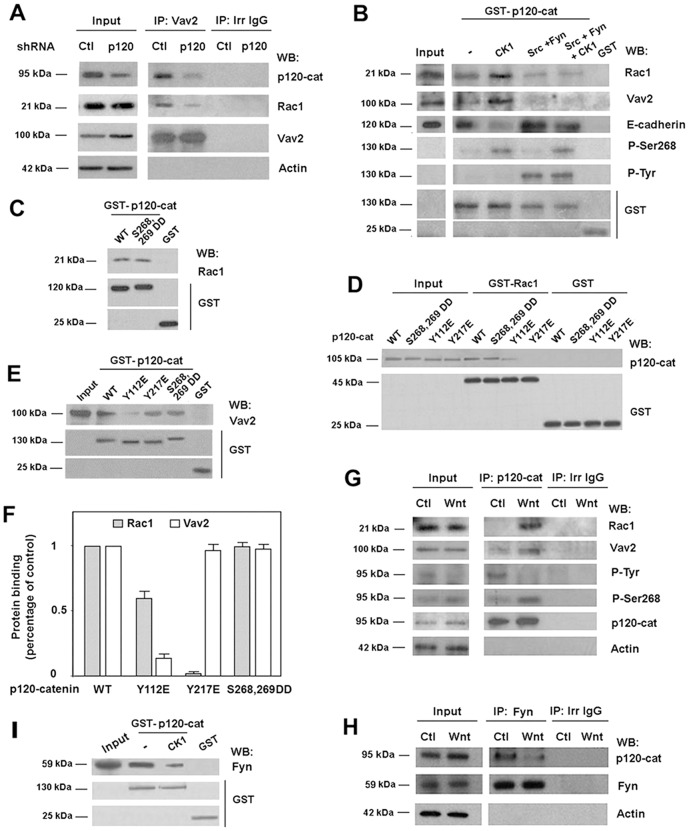
Fig. 3.
The direct interaction of p120-catenin with Rac1 and Vav2 is regulated by p120-catenin phosphorylation. (A) Vav2 was immunoprecipitated from 500 µg of control and p120-catenin (p120-cat)-depleted SW-480 cell extracts. Protein complexes were analyzed by western blot (WB) with anti-p120-catenin, anti-Rac1 and anti-Vav2. 5 µg of SW-480 whole-cell extracts were included as input. (B) GST–p120-catenin was phosphorylated by CK1, Src and Fyn at the times indicated and a pull-down assay was performed incubating 7 pmol of GST–p120-catenin or GST with cell extracts from SW-480 cells. Protein complexes were affinity purified and analyzed by WB. 4 µg of SW-480 lysate was included as input. (C) Full-length GST–p120-catenin wild type (wt), S 268,269 D (a p120-catenin mutant mimicking phosphorylated Ser268 and Ser269), or GST (2 pmol) were incubated with recombinant Rac1 (5 pmol). Protein complexes were affinity purified and analyzed by WB. (D) In vitro binding assays were performed by incubating GST–Rac1 or GST (5 pmol) with recombinant p120-catenin isoform 1 wt and p120-catenin mutants Y112E, Y217E (two Tyr→Glu mutants) or S268,269 D (Ser→Asp mutant) (1 pmol). Protein complexes were affinity purified and analyzed by WB with anti-p120-catenin and anti-GST mAbs. Internal reference standards (0.2 pmol) for p120-catenin wt and point mutants were included (St). (E) The isoform 1 of GST–p120-catenin wt and three specific point mutants (Y112E, Y217E and S268,S269D) (5 pmol) were incubated with HEK-293 cells lysates. Protein complexes were affinity purified and analyzed by WB with anti-Vav2 and anti-GST mAbs. 4 µg of HEK-293 lysate was included as input. (F) Autoradiograms from five different experiments performed in triplicate were quantified and the mean ± s.d. was obtained for each condition. The value of each mutant is presented relative to that obtained with p120-catenin WT construct. (G) p120-catenin was immunoprecipitated from 500 µg of SW-480 whole-cell extracts treated with control or Wnt3a-conditioned medium for 2 hours. 2 µg of SW-480 whole-cell extracts was included as input. Protein complexes were analyzed by WB. (H) Fyn was immunoprecipitated from 500 µg SW-480 whole-cell extracts treated with control or Wnt3a-conditioned medium for 2 hours. Protein complexes were analyzed by WB. 5 µg of SW480 lysate was included as input. (I) GST–p120-catenin (5 pmols) was phosphorylated by CK1 and incubated with SW-480 cell extracts. Protein complexes were affinity purified and analyzed by WB. 5 µg of SW-480 lysate was included as input.