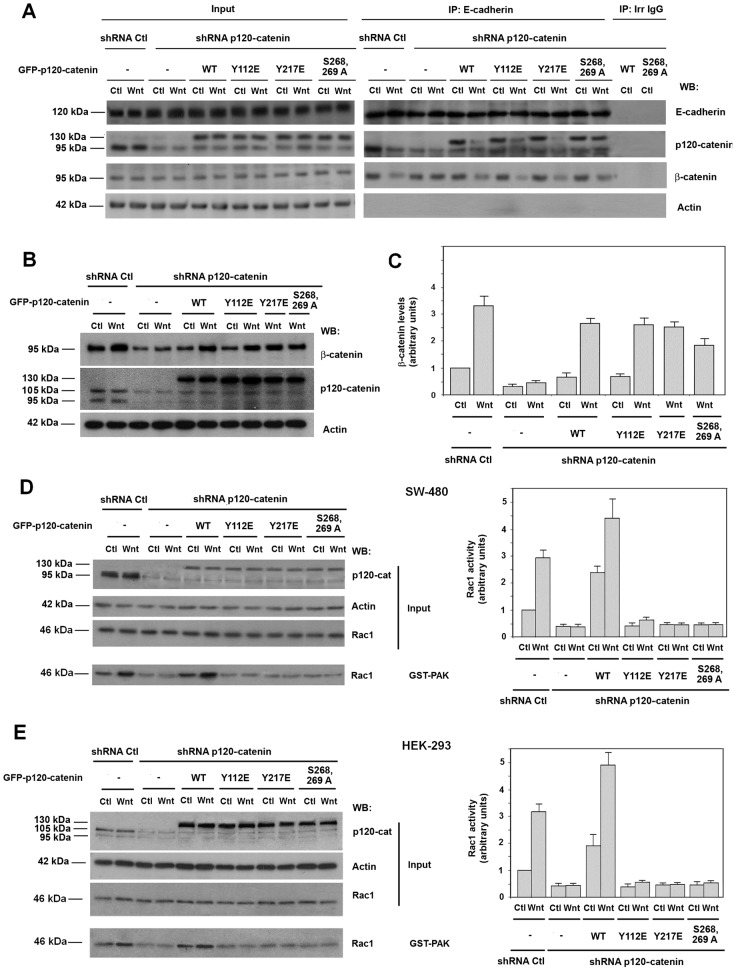
Fig. 5.
p120-catenin mutants unable to interact with Rac1 and Vav2 compromise Rac1 activation but not earlier steps of Wnt signaling. (A) E-cadherin was immunoprecipitated from control and p120-catenin (p120-cat)-depleted SW-480 cells overexpressing GFP–p120-catenin wild-type (wt) isoform 1, GFP–p120-catenin 1 point mutants Y112E, Y217E, S268,269A or the empty vector phrGFP and E-cadherin, and treated with control or Wnt3a-conditioned medium for 2 hours. Protein complexes were analyzed by western blot (WB) with the antibodies indicated. (B) Control and p120-catenin depleted HEK-293 cells overexpressing GFP–p120-catenin wt isoform 1, GFP–p120-catenin point mutants Y112E, Y217E and S268,269A or the empty vector phrGFP were treated with control or Wnt3a-conditioned medium for 9 hours and total β-catenin levels were analyzed by WB. (C) Autoradiograms from five different experiments performed as in B were quantified and the mean ± s.d. was obtained for each condition. Each value is presented relative to that obtained in nondepleted and nonstimulated cells. (D,E) p120-catenin-depleted SW-480 (D) or HEK-293 (E) cells overexpressing GFP–p120-catenin wt isoform 1, GFP–p120-catenin 1 harboring point mutants Y112E, Y217E, S268,269A and E-cadherin or the empty vector phrGFP were treated with control or Wnt3a-conditioned medium for 2 hours. GST–PAK pull-down assays were performed and active Rac1 was detected by WB. The graphs on the right show autoradiograms from five different experiments performed in triplicate) that were quantified and the mean ± s.d. obtained for each condition. Each value is presented relative to that obtained in nondepleted and nonstimulated cells.